Abstract
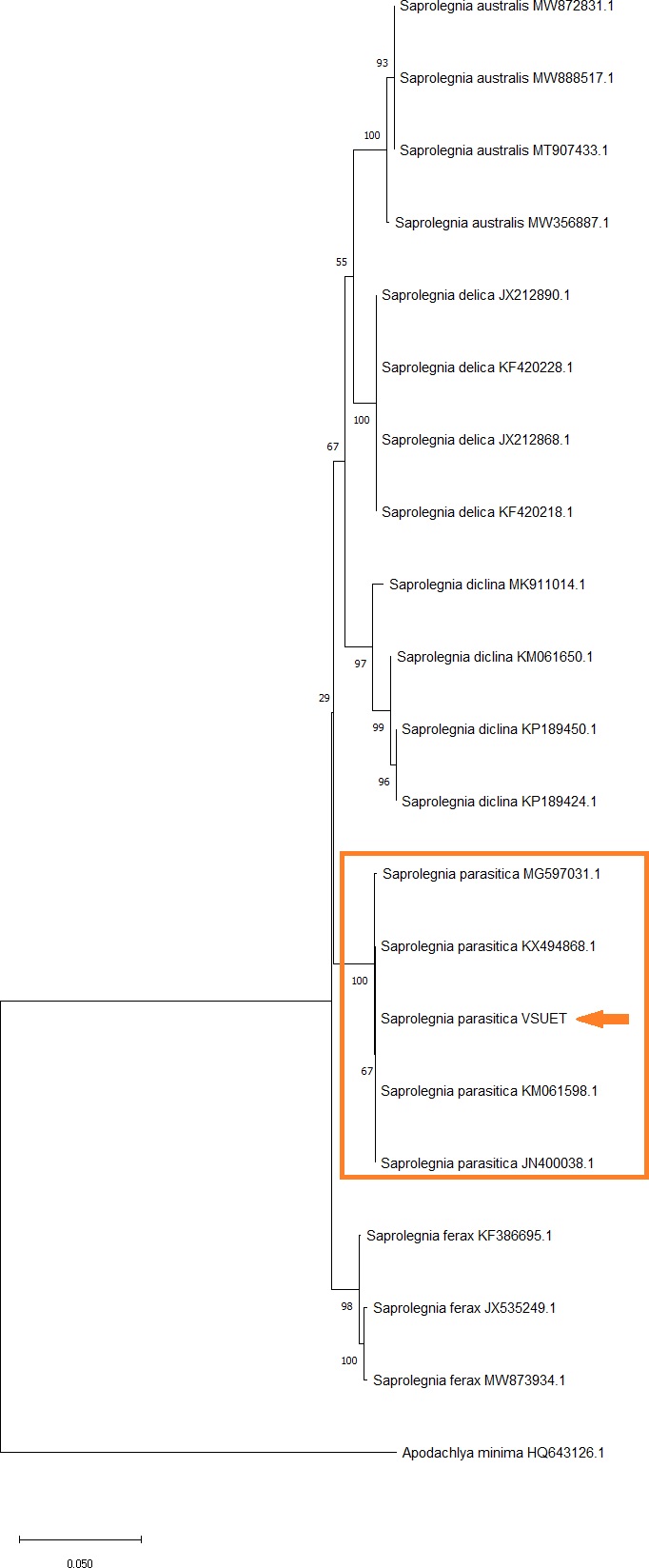
Saprolegniosis is considered one of the most common fungal diseases in freshwater aquaculture, affecting eggs and fish of all ages, and is causing great economic losses worldwide. In sturgeon aquaculture, highest harm is caused by caviar saprolegniosis (byssus), a mycotic disease of caviar, which is characterized by damage through saprolegnium fungi during hatchery incubation. The main infectious agents are aquatic mold fungi of the genus Saprolegnia spp. A sample of water mold was isolated from infected eggs of a hybrid of Russian sturgeon (Acipenser gueldenstaedtii) with kaluga (Huso dauricus) with characteristic signs of the disease. Microscopic examination of an isolated oomycete revealed morphological features characteristic of Saprolegnia spp., but no oogonia or antheridia were found, which complicates further species identification. To identify the isolated pathogen, molecular tools such as PCR and sequencing of a DNA section including 18S rRNA, ITS1, 5.8S rRNA, ITS2 and 28S rRNA were used to distinguish between different species of aquatic molds. Analysis of the obtained nucleotide sequence showed more than 99% identity with the previously known DNA sequences of S. parasitica. According to the results of phylogenetic analysis, the obtained nucleotide sequence was in the same group with the known sequences of S. parasitica and separated from other species belonging to S. ferax, S. diclina, S. delica, and S. australis.
References
Ali SE, Thoen E, Evensen O, Skaar I (2014) Boric acid inhibits germination and colonization of Saprolegnia spores in vitro and in vivo. PLoS One 9 (4): e91878. https://doi.org/10.1371/journal.pone.0091878
Bronzi P, Rosenthal H (2014) Present and future sturgeon and caviar production and marketing: A global market overview. Journal of Applied Ichthyology 30 (6): 1536–1546. https://doi.org/10.1111/jai.12628
Bronzi P, Chebanov M, Michaels JT, Wei Q, Rosenthal H, Gessner J (2019) Sturgeon meat and caviar production: Global update 2017. Journal of Applied Ichthyology 35: 257– 266. https://doi.org/10.1111/jai.13870
Ciulli S, Volpe E, Sirri R, Tura G, Errani F, Zamperin G, Toffan A, Silvi M, Renzi A, Abbadi M, Biasini L, Pretto T, Emmanuele P, Casalini A, Sarli G, Serratore P, Mordenti O, Mandrioli L (2020) Multifactorial causes of chronic mortality in juvenile sturgeon (Huso huso). Animals (Basel) 10: 1866. https://doi.org/10.3390%2Fani10101866
Eissa AE, Abdelsalam M, Tharwat N, Zaki M (2013) Detection of Saprolegnia parasitica in eggs of angelfish Pterophyllum scalare (Cuvier-Valenciennes) with a history of decreased hatchability. International Journal of Veterinary Science and Medicine 1: 7–14. https://doi.org/10.1016/j.ijvsm.2013.04.001
Ghiasi M, Khosravi AR, Soltani M, Binaii M, Shokri H, Tootian Z, Rostamibashman M, Ebrahimzademousavi H (2010) Characterization of Saprolegnia isolates from Persian sturgeon (Acipencer persicus) eggs based on physiological and molecular data. Journal of Medical Mycology 20: 1–7. https://doi.org/10.1016/j.mycmed.2009.11.005
González-Palacios C, Fregeneda-Grandes JM, Aller-Gancedo JM (2019) Biocontrol of saprolegniosis in rainbow trout (Oncorhynchus mykiss Walbaum) using two bacterial isolates (LE89 and LE141) of Pseudomonas fluorescens. Journal of Fish Diseases 42 (2): 269-275. https://doi.org/10.1111/jfd.12928
Heikkinen J, Tiirola M, Mustonen SM, Eskelinen P, Navia-Paldanius D, von Wright A (2016) Suppression of Saprolegnia infections in rainbow trout (Oncorhynchus mykiss) eggs using protective bacteria and ultraviolet irradiation of the hatchery water. Aquaculture Research 47 (3): 925–939. https://doi.org/10.1111/are.12551
Hu K, Ma RR, Cheng JM, Ye X, Sun Q, Yuan HL, Liang N, Fang WH, Li HR, Yang XL (2016) Analysis of Saprolegnia parasitica transcriptome following treatment with copper sulfate. PLoS One 11 (2): e0147445. https://doi.org/10.1371/journal.pone.0147445
Jalilpoor J, Mosouleh SA, Masoumzadeh M (2006) Fungal flora in Acipenser persicus eggs with particular emphasis on Saprolegnia sp. (Oomycetes) and mortality during mass incubation at the Shahid Behesti hatchery. Journal of Applied Ichthyology 22: 265–268. https://doi.org/10.1111/j.1439-0426.2007.00965.x
Ke XL, Wang JG, Gu ZM, Li M, Gong XN (2009) Morphological and molecular phylogenetic analysis of two Saprolegnia sp. (Oomycetes) isolated from silver crucian carp and zebra fish. Mycological Research 113 (5): 637–644. https://doi.org/10.1016/j.mycres.2009.01.008
Liu Y, Rzeszutek E, van der Voort M, Wu CH, Thoen E, Skaar I, Bulone V, Dorrestein P C, Raaijmakers JM, de Bruijn1 I (2015) Diversity of aquatic Pseudomonas species and their activity against the fish pathogenic oomycete Saprolegnia. PLoS One 10(8): e0136241. https://doi.org/10.1371%2Fjournal.pone.0136241
Madrid A, Godoy P, González S, Zaror L, Moller A, Werner E, Cuellar M, Villena J, Montenegro I (2015) Chemical characterization and anti-oomycete activity of Laureliopsis philippianna essential oils against Saprolegnia parasitica and S. australis. Molecules 20 (5): 8033–8047. https://doi.org/10.3390%2Fmolecules20058033
Radosavljevic V, Milicevic V, Maksimović-Zorić J, Veljović L, Nesic K, Pavlović M, Ljubojević-Pelić D, Marković Z (2019) Sturgeon diseases in aquaculture. Archivos de Medicina Veterinaria 12 (1): 5–20. https://doi.org/10.46784/e-avm.v12i1.34
Sandoval-Sierra JV, Diéguez-Uribeondo J (2015) A comprehensive protocol for improving the description of Saprolegniales (Oomycota): two practical examples (Saprolegnia aenigmatica sp. nov. and Saprolegnia racemosa sp. nov.). PLoS One 10 (7): e0132999. https://doi.org/10.1371/journal.pone.0132999
Sandoval-Sierra JV, Martín MP, Diéguez-Uribeondo J (2014) Species identification in the genus Saprolegnia (Oomycetes): defining DNA-based molecular operational taxonomic units. Fungal Biology 118 (7): 559–578. https://doi.org/10.1016/j.funbio.2013.10.005
Sandoval-Sierra JV, Latif-Eugenin F, Martín MP, Zaror L, Diéguez-Uribeondo J (2014) Saprolegnia species affecting the salmonid aquaculture in Chile and their associations with fish developmental stage. Aquaculture 434: 462–469. https://doi.org/10.1016/j.aquaculture.2014.09.005
Shin S, Kulatunga DCM, Dananjaya SHS, Nikapitiya C, Lee J, De Zoysa M (2017) Saprolegnia parasitica isolated from rainbow trout in Korea: characterization, anti-saprolegnia activity and host-pathogen interaction in zebrafish disease model. Mycobiology 45 (4): 297–311. https://doi.org/10.5941/myco.2017.45.4.297
Songe MM, Willems A, Wiik‐Nielsen J, Thoen E, Evensen O, van West P, Skaar I (2016) Saprolegnia diclina IIIA and S. parasitica employ different infection strategies when colonizing eggs of Atlantic salmon, Salmo salar L. Journal of Fish Diseases 39 (3): 343–352. https://doi.org/10.1111/jfd.12368
Tedesco P, Fioravanti ML, Galuppi R (2019) In vitro activity of chemicals and commercial products against Saprolegnia parasitica and Saprolegnia delica strains. Journal of Fish Diseases 42: 237–248. https://doi.org/10.1111/jfd.12923
Tedesco P, Beraldo P, Massimo M, Fioravanti ML, Volpatti D, Dirks R, Galuppi R (2020) Comparative therapeutic effects of natural compounds against Saprolegnia spp. (Oomycota) and Amyloodinium ocellatum (Dinophyceae). Frontiers in Veterinary Science 7: 83. https://doi.org/10.3389/fvets.2020.00083
Thoen E, Evensen O, Skaar I (2011) Pathogenicity of Saprolegnia spp. to Atlantic salmon, Salmo salar L., eggs. Journal of Fish Diseases 34: 601–608. https://doi.org/10.1111/j.1365-2761.2011.01273.x
Acta Biologica Sibirica is a golden publisher, as we allow self-archiving, but most importantly we are fully transparent about your rights.
Authors may present and discuss their findings ahead of publication: at biological or scientific conferences, on preprint servers, in public databases, and in blogs, wikis, tweets, and other informal communication channels.
ABS allows authors to deposit manuscripts (currently under review or those for intended submission to ABS) in non-commercial, pre-print servers such as ArXiv.
Authors who publish with this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License (CC BY 4.0) that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgement of its initial publication in this journal.
- Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See The Effect of Open Access).