SUPRAMOLECULAR COMPLEXES OF GLYCYRRHISIC ACID AND ITS MONOAMMONIUM SALT WITH ETHACISIN HYDROCHLORIDE
UDC 547.918:547.551:543.42
Abstract
The problem of effective and safe therapy of cardiac arrhythmias requires the creation of new antiarrhythmic drugs with low toxicity. Clathration of medicinal substances with cyclodextrins or plant glycosides is a promising method for reducing their side effects and increasing their solubility.
The aim of this research is the complex formation of ethacizin hydrochloride with triterpene glycosides and the study of physicochemical properties of the resulting complexes.
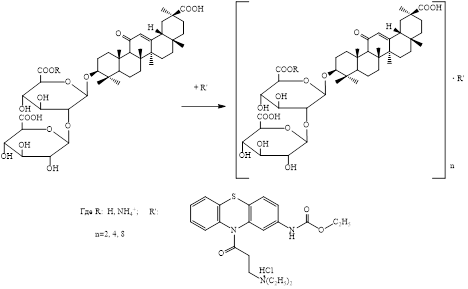
Complexes of glycyrrhizic acid (GA) and its monoammonium salt (MASGA) with ethacizine hydrochloride (EtHQ) in various molar ratios (2 : 1, 4 : 1, 8 : 1) characterized by certain physicochemical parameters have been obtained. The resulting inclusion compounds were studied by UV and IR spectroscopy. It has been determined that molecular complexes of GA and MASGA with EtHQ are formed through weak intermolecular interactions, such as hydrogen bonding, electrostatic and hydrophobic interactions. Supramolecular complexes of natural glycosides with EtHQ have the same stoichiometric composition, approximately equal to the stability constant, and the negative value of the Gibbs free energy confirms the formation of a molecular complex as a result of autoassociation.
The obtained experimental data can serve and/or supplement scientific data for the creation of new promising drugs with a broad therapeutic effect, targeted delivery, synergistic effect and low toxicity.
Downloads
Metrics
References
Conen D., Adam M., Roche F., Barthelemy J.C. et al. Circulation, 2012, vol. 126, pp. 2302–2308. DOI: 10.1161/CIRCULATIONAHA.112.112300.
Zatonskaya Ye.V., Matyushin G.V., Gogolashvvili N.G., Novgoroldtseva N.Ya. Sibirskoye meditsinskoye obozreniye, 2016, no. 3, pp. 5–16. (in Russ.).
Pelinovskaya L.I., Demko I.V., Mandrikova O.M., Glizer R.N. Sibirskoye meditsinskoye obozreniye, 2013, no. 4, pp. 60–62. (in Russ.).
Murakoshi N., Aonuma K. Circ. J., 2013, vol. 77, pр. 2419–2431. DOI: 10.1253/circj.cj-13-1129.
Zatonskaya Ye.V., Matyushin G.V., Gogolashvili N.G. Ratsional'naya farmakoterapiya v kardiologii, 2017, no. 13(3), pp. 403–408. (in Russ.).
Bussink E., Holst A.G., Jespersen L., Deckers J.W., Jensen G.B., Prescott E. Eur. Heart J., 2013, vol. 34, pр. 138–146. DOI: 10.1093/eurheartj/ehs291.
Jensen P.N., Gronroos N.N., Chen L.Y., Folsom A.R., de Filippi C., Heckbert S.R., Alonso A. J. Am. Coll. Cardiol., 2014, vol. 64, pp. 5315–5338. DOI: 10.1016/j.jacc.2014.03.056.
Zatonskaya Ye.V., Matyushin G.V., Gogolashvili N.G., Novgorodtseva N.Ya., Shul'min A.V. Sibirskoye med-itsinskoye obozreniye, 2015, no. 4, pp. 52–56. (in Russ.).
Zatonskaya Ye.V., Matyushin G.V., Gogolashvili N.G., Novgorodtseva N.Ya., Shul'min A.V. Sibirskoye med-itsinskoye obozreniye, 2015, no. 3, pp. 74–78. (in Russ.).
Malakhov V.I., Golitsyn S.P., Sokolov S.F., Smetnev A.S. Byulleten' VKNTs, 1987, no. 3, pp. 112–116. (in Russ.).
Tsaregorodtsev D.A. Rossiyskiy kardiologicheskiy zhurnal, 2001, no. 2, pp. 68–75. (in Russ.).
Tolstikova T.G., Tolstikov A.G., Tolstikov G.A. Vestnik RAN, 2007, vol. 77, no. 10, pp. 867–874. (in Russ.).
Tolstikov G.A., Baltina L.A., Grankina V.P., Kondratenko R.M., Tolstikova T.G. Solodka bioraznoobraziye, khimiya, primeneniye v meditsine. [Licorice biodiversity, chemistry, medicinal uses]. Novosibirsk, 2007, 311 p. (in Russ.).
Polyakov N.E., Leshina T.V. Open Conf. Proc. J., 2011, vol. 2, pр. 64–72. DOI: 10.2174/2210289201102010064.
Hostettmann K., Marston A. Saponins. Cambrige: Cambrige University Press, 1995, 548 p. DOI: 10.1017/CBO9780511565113.
Sasaki Y., Mizutani K., Kasai R., Tanaka O. Chem. Pharm. Bull., 1988, vol. 36, pр. 3491–3495.
Gilbert R.J., James K.C. J. Pharm. Pharmacol., 1964, vol. 16, pp. 394–399. DOI: 10.1111/j.2042-7158.1964.tb07481.x.
Krasova T.G., Bashura G.S., Muravev I.A. Farmatsiya, 1978, vol. 27, no. 5, pp. 32–35. (in Russ.).
Baltina L.A., Kondratenko R.M., Mustafina S.R. i dr. Khimiko-farmatsevticheskiy zhurnal, 2001, vol. 35, no. 1, pp. 38–41. (in Russ.).
Yuldashev Kh.A., Mukhamediyev M.G., Dalimov D.N. i dr. Khimiya i khimicheskaya tekhnologiya, 2011, no. 1, pp. 24–26. (in Russ.).
Matchanov A.D., Esanov R.S., Renkawitz T. et al. Materials, 2022, vol. 15, p. 4197. DOI: 10.3390/ma15124197.
Yakovishin L.A., Grishkovets V.I., Korzh Ye.N. Uchenyye zapiski natsional'nogo universiteta im V.I. Vernadskogo, 2014, vol. 27(66), no. 4, pp. 131–137. (in Russ.).
Borisenko S.N., Lekar' A.V., Milov A.A. i dr. Khimiya rastitel'nogo syr'ya, 2013, no. 2, pp. 85–92. DOI: 10.14258/jcprm.2016031175. (in Russ.).
Tykarska E., Gdaniec M. Cryst. Growth Des., 2013, vol. 13, pp. 1301−1308. DOI: 10.1021/cg301768h.
Tykarska E., Sobiak S., Gdaniec M. Crystal Growth & Desgign, 2012, vol. 12, pp. 2133−2137. DOI: 10.1021/cg300160c.
Romanko T.V., Murinov Yu.I. Zhurnal fizicheskoy khimii, 2001, vol. 75, no. 9, pp. 1601–1604. (in Russ.).
Babko A.K. Fiziko-khimicheskiy analiz kompleksnykh soyedineniy v rastvorakh. [Physicochemical analysis of complex compounds in solutions]. Kiev, 1955, 328 p. (in Russ.).
Roy B., Saha A., Esterrani A., Nandi A.K. Soft Matter, 2010, vol. 6, pp. 3337–3345. DOI: 10.1039/C0SM00036A.
Bulatov M.I., Kalinkin I.P. Prakticheskoye rukovodstvo po fotometricheskim metodam analiza. [Practical guide to pho-tometric methods of analysis]. Leningrad, 1986, 432 p. (in Russ.).
Yakovishin L.A. Molekulyarnyye kompleksy triterpenovykh glikozidov s biologicheski aktivnymi veshchestvami: polu-cheniye, khimiko-farmatsevticheskiye svoystva i biologicheskaya aktivnost': diss. … dokt. khim. nauk. [Molecular com-plexes of triterpene glycosides with biologically active substances: preparation, chemical-pharmaceutical properties and biological activity: dissertation. ... doc. chem. Sci.]. Sevastopol', 2018, 351 p. (in Russ.).
Copyright (c) 2023 chemistry of plant raw material

This work is licensed under a Creative Commons Attribution 4.0 International License.

This work is licensed under a Creative Commons Attribution 4.0 International License.
The authors, which are published in this journal, agree to the following conditions:
1. Authors retain the copyright to the work and transfer to the journal the right of the first publication along with the work, at the same time licensing it under the terms of the Creative Commons Attribution License, which allows others to distribute this work with the obligatory indication of the authorship of this work and a link to the original publication in this journal .
2. The authors retain the right to enter into separate, additional contractual agreements for the non-exclusive distribution of the version of the work published by this journal (for example, to place it in the university depository or to publish it in a book), with reference to the original publication in this journal.
3. Authors are allowed to post their work on the Internet (for example, in a university repository or on their personal website) before and during the review process of this journal, as this may lead to a productive discussion, as well as more links to this published work.











