STUDY OF THE MECHANISM OF SORPTION OF Cu2+, Co2+, AND Mn2+ IONS ON A MODIFIED NATURAL POLYMER – PECTIN
UDК 66.081.2:544.723.5
Abstract
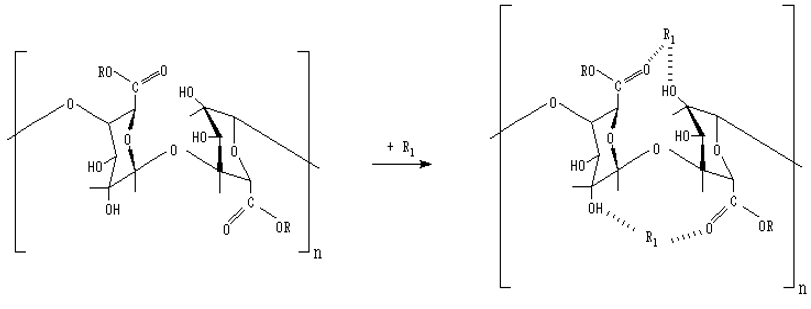
Sorption materials based on apple pectin modified with biologically active organic acids (salicylic, anthranilic, 5-aminosalicylic, nicotinic) capable of effectively extracting Cu2+, Co2+ and Mn2+ ions from aqueous solutions of their salts have been obtained. The regularities of the sorption kinetics of Cu2+, Co2+ and Mn2+ ions by modified pectin samples were studied. An increase in the efficiency of extraction of d-metal ions by modified pectin sorbents compared to the original polysaccharide was revealed. Sorption isotherms of Cu2+, Co2+, Mn2+ ions by modified pectins have been obtained and analyzed for compliance with known theoretical models. Integral kinetic curves have been obtained, and the values of the experimental sorption capacity of biosorbents have been calculated. It was found that the process of sorption of Cu2+, Co2+ and Mn2+ ions on the studied biosorbents proceeds in the diffusion mode. The predominance of the external diffusion nature of the limiting stage of the process of extracting transition metal ions with modified pectins was established. The rate constants of sorption processes are calculated. The values of the apparent activation energy of the sorption process are determined. The thermodynamic parameters of the extraction of Cu2+, Co2+ and Mn2+ ions by pectin sorbents have been calculated. It has been established that the sorption of Cu2+, Co2+, and Mn2+ ions by modified polysaccharide materials is an exothermic process, which can be considered as physical adsorption of metal ions due to solvation and complex formation with the participation of sorbent sorption centers and solvent (water) molecules. The obtained new highly active biosorbents can be recommended as enterosorbents for detoxification of the human body.
Keywords: sorption, d-metal ions, pectin, modification, kinetic curves, thermodynamic parameters.
Downloads
Metrics
References
Kaysheva N.Sh., Kayshev A.Sh. Farmakokhimicheskiye osnovy primeneniya pektinov i al'ginatov. [Pharmacochemical basis for the use of pectins and alginates]. Pyatigorsk, 2016, 260 p. (in Russ.).
Zhang W., Song J., He Q., Wang H., Lyu W., Feng H., Chen L. Journal of Hazardous Materials, 2020, vol. 384, 121445. DOI: 10.1016/j.jhazmat.2019.121445.
Liang R., Li P., He X., Kuang M., Chen J., Liu C. Science Technology of Food Industry, 2018, vol. 39, no. 6, pp. 13–18.
Karmakar M., Mondal H., Mahapatra M., Chattopadhyay P.K., Chatterjee S., Singha N.R. Carbohydrate Polymers, 2019, vol. 206, pp. 778–791. DOI: 10.1016/j.carbpol.2018.11.032.
Kaushal S., Kaur N., Kaur M., Singh P.P. Journal of Photochemistry and Photobiology A: Chemistry, 2020, vol. 403, 112841. DOI: 10.1016/j.jphotochem.2020.112841.
Kodoth A.K., Badalamoole V. Polymer Bulletin, 2020, vol. 77, no. 2, pp. 541–564. DOI: 10.1007/s00289-019-02757-4.
Martins J.G., Facchi D.P., Berton S.B.R., Nunes C.S., Matsushita M., Bonafe E.G., Martins A.F. International Jour-nal of Biological Macromolecules, 2020, vol. 152, pp. 77–89. DOI: 10.1016/j.ijbiomac.2020.02.220.
Shao Z., Lu J., Ding J., Fan F., Sun X., Li P., Hu Q. International Journal of Biological Macromolecules, 2021, vol. 176, pp. 217–225. DOI: 10.1016/j.ijbiomac.2021.02.037.
Wang X.D., Li Y., Dai T.T., He X.M., Chen M.S., Liu C.M., Liang R.H., Chen J. Carbohydrate Polymers, 2021, vol. 260, 117811. DOI: 10.1016/j.carbpol.2021.117811.
Liang R., Li Ya, Li H., Wang X., Hu X., Liu C., Chen M., Chen J. Carbohydrate Polymers, 2020, vol. 234, 115911. DOI: 10.1016/j.carbpol.2020.115911.
Zhu W., Yang J., Hu D., Wang Z. Food & Function, 2021, vol. 12, no. 6, pp. 2418–2427. DOI: 10.1039/d0fo02829k.
Li J., Yang Z., Ding T., Song Y.-J. Carbohydrate Polymers, 2022, vol. 276, 118789. DOI: 10.1016/j.carbpol.2021.118789.
Kupchik L.A., Kartel’ N.T., Bogdanov E.S., Bogdanova O.V., Kupchik M.P. Russian Journal of Applied Chemistry, 2006, vol. 79, no. 3, pp. 457–460. DOI: 10.1134/S1070427206030256.
Li F.T., Yang H., Zhao Y., Xu R. Chinese Chemical Letters, 2007, vol. 18, pp. 325–328. DOI: 10.1016/j.cclet.2007.01.034.
Arachchige M.P., Mu T., Ma M. Chemosphere, 2021, vol. 262, 128102. DOI: 10.1016/j.chemosphere.
Praveen S., Kshipra S., Pankaj T., Manoj C., Kalpana C. International Journal of Biological Macromolecules, 2019, vol. 140, pp. 78–90.
Eliaz I.., Weil E., Wilk B. Forsch Komplementmed, 2007, vol. 14, pp. 358–364. DOI: 10.1159/000109829.
Eliaz I., Hotchkiss A.T., Fishman M.L., Rode D. Phytotherapy Research, 2006, vol. 20, pp. 859–864. DOI: 10.1002/ptr.1953.
Bhuyan M., Okabe H., Hidaka Y., Hara K. Journal of Applied Polymer Science, 2018, vol. 135, p. 8. DOI: 10.1002/app.20171479.
Gong J.-L., Wang X.-Y., Zeng G-M., Chen L. Chemical Engineering Journal, 2012, vol. 185–186, pp. 100–107. DOI: 10.1016/j.cej.2012.01.050.
Mudarisova R.Kh., Sagitova A.F., Kukovinets O.S. Fiziko-khimiya poverkhnosti i zashchita materialov, 2022, vol. 58, no. 5, pp. 480–488. DOI: 10.31857/S0044185622050175. (in Russ.).
Mudarisova R., Kukovinets О., Sagitova А., Novoselov I. Biointerface Research in Applied Chemistry, 2020, vol. 10, no. 4, pp. 5724–5732. DOI: 10.33263/BRIAC104.724732.
Muginova S.V. Metodicheskiye ukazaniya k kursu analiticheskoy khimii. [Guidelines for the course of analytical chem-istry]. Moscow, 2007, 81 p. (in Russ.).
Gayduk O.V., Pantaler R.P. Analitika i kontrol', 2010, vol. 14, no. 1, pp. 25–28. (in Russ.).
Hawari A., Rawajfih Z., Nsour N. Journal of Hazardous Materials, 2009, vol. 168, pp. 1284–1289. DOI: 10.1016/j.jhazmat.2009.03.014.
Ho Y.S., Ng J.C., McKay G. Separation and Purification Methods, 2000, vol. 29, no. 2, pp. 189–232.
Lazaridis N.K., Karapantsios D., Georgantas D. Water Research, 2003, vol. 37, pp. 3023–3033. DOI: 10.1016/S0043-1354(03)00121-0.
Kokotov Yu.A, Zolotarev G.E., Yel'kin. P.P. Teoreticheskiye osnovy ionnogo obmena: Slozhnyye ionoobmennyye sis-temy. [Theoretical foundations of ion exchange: Complex ion exchange systems]. Leningrad, 1986, 280 p. (in Russ.).
Boyd G.E., Adamson A.W., Myers L.S. Journal of the American Chemical Society, 1947, vol. 69, no. 10, pp. 2836–2848.
Gel'ferikh F. Ionity: Osnovy ionnogo obmena. [Ion exchangers: Fundamentals of ion exchange]. Moscow, 1962, 490 p. (in Russ.).
Farooq U., Kozinski J.A. Bioresource Technology, 2010, vol. 101, pp. 5043–5053.
Schiewer S. Journal of Hazardous Materials, 2008, vol. 157, pp. 8–17. DOI: 10.1016/j.jhazmat.2007.12.076.
Reddy N.S., Rao K.M., Vani T.J.S., Rao K.S.V.K., Lee Y.I. Desalination and Water Treatment, 2016, vol. 57, no. 14, pp. 1–12. DOI: 10.1080/19443994.2015.1008053.
Copyright (c) 2024 chemistry of plant raw material

This work is licensed under a Creative Commons Attribution 4.0 International License.

This work is licensed under a Creative Commons Attribution 4.0 International License.
The authors, which are published in this journal, agree to the following conditions:
1. Authors retain the copyright to the work and transfer to the journal the right of the first publication along with the work, at the same time licensing it under the terms of the Creative Commons Attribution License, which allows others to distribute this work with the obligatory indication of the authorship of this work and a link to the original publication in this journal .
2. The authors retain the right to enter into separate, additional contractual agreements for the non-exclusive distribution of the version of the work published by this journal (for example, to place it in the university depository or to publish it in a book), with reference to the original publication in this journal.
3. Authors are allowed to post their work on the Internet (for example, in a university repository or on their personal website) before and during the review process of this journal, as this may lead to a productive discussion, as well as more links to this published work.











