SYNTHESIS OF GLYCIVIR DERIVATIVES USING MODIFICATION OF SYNTHESIS PROCEDURE STUDYING THEIR ANTIVIRAL ACTIVITY AGAINST ENV-PSEUDOVIROUSES HIV-1
UDC 578.233.33+578.233.36
Abstract
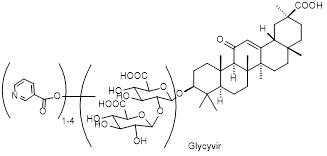
HIV infection still remains a global health problem around the world. The fight against infection is carried out both through preventive measures and timely testing for the presence of HIV and the use of antiretroviral therapy (ART) when it is detected. Researchers are constantly actively searching for new medicinal agents. In this work, new modified methods for the synthesis of glycivir were tested, including changing the holding time of the reaction mixture, varying the amount of starting reagents, adding catalysts, replacing the solvent, and replacing the condensing agent. Ten variants of glycivir were obtained, for each of which, using the MTT test, a 50% cytotoxic concentration was determined against the TZM-bl cell line and antiviral activity on the model of HIV-1 env-pseudoviruses. Sample 10 had the greatest activity against HIV-1 env-pseudoviruses, the synthesis of which involved the complete replacement of phosphorus and pyridine pentachloride with more accessible and less toxic methyl chloroformate and triethylamine in chloroform. These changes made to the original method for the synthesis of glycivir make it possible to obtain a drug that is most similar in biological activity to glycivir, but at the same time replace highly toxic reagents during the synthesis with less toxic and cheaper ones.
Downloads
Metrics
References
Tompa D.R., Immanuel A., Srikanth S., Kadhirvel S. International journal of biological macromolecules, 2021, vol. 172, pp. 524–541. DOI: 10.1016/j.ijbiomac.2021.01.076.
Phanuphak N., Gulick R.M. Current Opinion in HIV and AIDS, 2020, vol. 15, pp. 4–12. DOI: 10.1097/COH.0000000000000588.
Arts E.J., Hazuda D.J. Cold Spring Harbor perspectives in medicine, 2012, vol. 2(4), a007161. DOI: 10.1101/cshperspect.a007161.
Yarovaya O.I., Salakhutdinov N.F. Russian Chemical Reviews, 2021, vol. 90, p. 488. DOI: 10.1070/RCR4969.
Musarra-Pizzo M., Pennisi R., Ben-Amor I., Mandalari G., Sciortino M.T. Viruses, 2021, vol. 13, p. 828. DOI: 10.3390/v13050828.
Hirabayashi K., Iwata S., Matsumoto H., Mori T., Shibata S., Baba M., Ito M., Shigeta S., Nakashima H., Yamamo-to N. Chemical and pharmaceutical bulletin, 1991, vol. 39, pp. 112–115. DOI: 10.1248/cpb.39.112.
Ji B., Zhao Y., Yu P., Yang B., Zhou C., Yu Z. Talanta, 2018, vol. 190, pp. 450–459. DOI: 10.1016/j.talanta.2018.08.020.
Sokolova A.S., Putilova V.P., Yarovaya O.I., Zybkina A.V., Mordvinova E.D., Zaykovskaya A.V., Shcherba-kov D.N., Orshanskaya I.R., Sinegubova E.O.,. Esaulkova I.L, Borisevich S.S., Bormotov N.I., Shishkina L.N., Za-rubaev V.V., Pyankov O.V. Molecules, 2021, vol. 26, p. 2235. DOI: 10.3390/molecules26082235.
Rex R.S., Nadar M.S., Selvakumar P.M. BiorgOrgChem, 2018, vol. 2, pp. 61–70. DOI: 10.15406/mojboc.2018.02.00058.
Ghildiyal R., Prakash V., Chaudhary V.K., Gupta V., Gabrani R. Plant-derived Bioactives. Springer, Singapore, 2020, pp. 279–295. DOI: 10.1007/978-981-15-1761-7_12.
Baltina L.A., Kondratenko R.M., Baltina (ml) L.A., Plyasunova O.A., Pokrovskiy A.G., Tolstikov G.A. Khimiko-farmatsevticheskiy zhurnal, 2009, vol. 43, no. 10, pp. 3–12. DOI: 10.30906/0023-1134-2009-43-10-3-12. (in Russ.).
Ito M., SatoA., Hirabayashi K., Tanabe F., Shigeta S., Baba M., Clercq E.D., Nakashima H., Yamamoto N. Antiviral Research, 1988, vol. 10, pp. 289–298. DOI: 10.1016/0166-3542(88)90047-2.
Zarubayev V.V., Anikin V.B., Smirnov V.S. Infektsiya i immunitet, 2016, vol. 6, no. 3, pp. 199–206. (in Russ.).
Kondratenko P.M., Baltina L.A., Mustafina SR., Vasil'yeva Ye.V., Pompei R., Deydda D., Plyasunova O.A., Po-krovskiy A.G., Tolstikov G.A. Bioorganicheskaya khimiya, 2004, vol. 30, no. 3, pp. 308–315. (in Russ.).
Hoever G., Baltina L., Michaelis M., Kondratenko R., Baltina L. (Jr.), Tolstikov G.A., Doerr H.W., Cinatl J. (jr.). J. Med. Chem., 2005, vol. 48, pp. 1256–1259. DOI: 10.1021/jm0493008.
Patent 2299740 (RU). 2007. (in Russ.).
Patent 2199547 (RU). 2003. (in Russ.).
Patent 2304145 (RU). 2007. (in Russ.).
Fomenko V.V., Rudometova N.B., Yarovaya O.I., Rogachev A.D., Fando A.A., Zaykovskaya A.V., Komarova N.I., Shcherbakov D.N., Pyankov O.V., Pokrovsky A.G., Karpenko L.I., Maksyutov R.A., Salakhutdinov N.F. Molecules, 2022, vol. 27, p. 295. DOI: 10.3390/molecules27010295.
Kovaleva K.S., Yarovaya O.I., Gatilov Yu.V., Slita A.V., Yesaulkova Ya.L., Zarubayev V.V., Rudometova N.B., Shcherbakova N.S., Shcherbakov D.N., Salakhutdinov N.F. Khimiya geterotsiklicheskikh soyedineniy, 2021, vol. 57, no. 4, pp. 455–461. (in Russ.).
Revilla A., Delgado E., Christian E.C., Dalrymple J., Vega Y., Carrera C., González-Galeano M., Ocampo A., de Cas-tro R.O., Lezaún M.J., Rodríguez R., Mariño A., Ordóñez P., Cilla G., Cisterna R., Santamaría J.M., Prieto S., Rakh-manova A., Vinogradova A., Ríos M., Pérez-Álvarez L., Nájera R., Montefiori D.C., Seaman M.S., Thomson M.M. AIDS Res. Hum. Retroviruses, 2011, vol. 27, pp. 889–901. DOI: 10.1089/aid.2010.0177.
Domingo P., de Benito N. EBioMedicine, 2021, vol. 74, 103703. DOI: 10.1016/j.ebiom.2021.103703.
Legnani L., Colombo D., Venuti A., Pastori C., Lopalco L., Toma L., Mori M., Grazioso G., Villa S. Med. Chem. Commun., 2017, vol. 8, pp. 422–433. DOI: 10.1039/C6MD00575F.
Baram G.I., Grachev M.A., Komarova N.I., Perelroyzen M.P., Bolvanov Yu. A., Kuzmin S.V., Kargaltsev V.V., Kuper E.A. Journal of Chromatography A, 1983, vol. 264, pp. 69–90. DOI: 10.1016/S0021-9673(01)95007-1.
Ladinsky M.S., Gnanapragasam P.N., Yang Z., West A.P., Kay M.S., Bjorkman P.J. Elife, 2020, vol. 9, e58411. DOI: 10.7554/eLife.58411.
Zaitsev B.N., Taranov O.S., Rudometova N.B., Shcherbakova N.S., Ilyichev A.A., Karpenko L.I. Vavilov Journal of Genetics and Breeding, 2019, vol. 23, pp. 337–342. DOI: 10.18699/VJ19.498.
Dando T.M., Perry C.M. Drugs, 2003, vol. 63, pp. 2755–2766. DOI: 10.2165/00003495-200363240-00005.
Lalezari J.P., Henry K., O'Hearn M., Montaner J.S.G., Piliero P.J.,Trottier B., Walmsley S., Cohen C., Kuritzkes D.R., Eron J.J. Jr., Chung J., DeMasi R., Donatacci L., Drobnes C., Delehanty J., Salgo M. New England Journal of Medi-cine, 2003, vol. 348, pp. 2175–2185. DOI: 10.1056/NEJMoa035026.
Kitchen C.M.R., Nuño M., Kitchen S.G., Krogstad P. Therapeutics and clinical risk management, 2008, vol. 4, pp. 433–439. DOI: 10.2147/tcrm.s1962.
Clercq E.D. International Journal of Antimicrobial Agents, 2009, vol. 33, pp. 307–320. DOI: 10.1016/j.ijantimicag.2008.10.010.
Copyright (c) 2023 chemistry of plant raw material

This work is licensed under a Creative Commons Attribution 4.0 International License.

This work is licensed under a Creative Commons Attribution 4.0 International License.
The authors, which are published in this journal, agree to the following conditions:
1. Authors retain the copyright to the work and transfer to the journal the right of the first publication along with the work, at the same time licensing it under the terms of the Creative Commons Attribution License, which allows others to distribute this work with the obligatory indication of the authorship of this work and a link to the original publication in this journal .
2. The authors retain the right to enter into separate, additional contractual agreements for the non-exclusive distribution of the version of the work published by this journal (for example, to place it in the university depository or to publish it in a book), with reference to the original publication in this journal.
3. Authors are allowed to post their work on the Internet (for example, in a university repository or on their personal website) before and during the review process of this journal, as this may lead to a productive discussion, as well as more links to this published work.











