SYNTHESIS AND ANTIRADICAL ACTIVITY OF HINDERED PHENOLIC DERIVATIVES OF FLAX CELLULOSE
UDC 661.728.89
Abstract
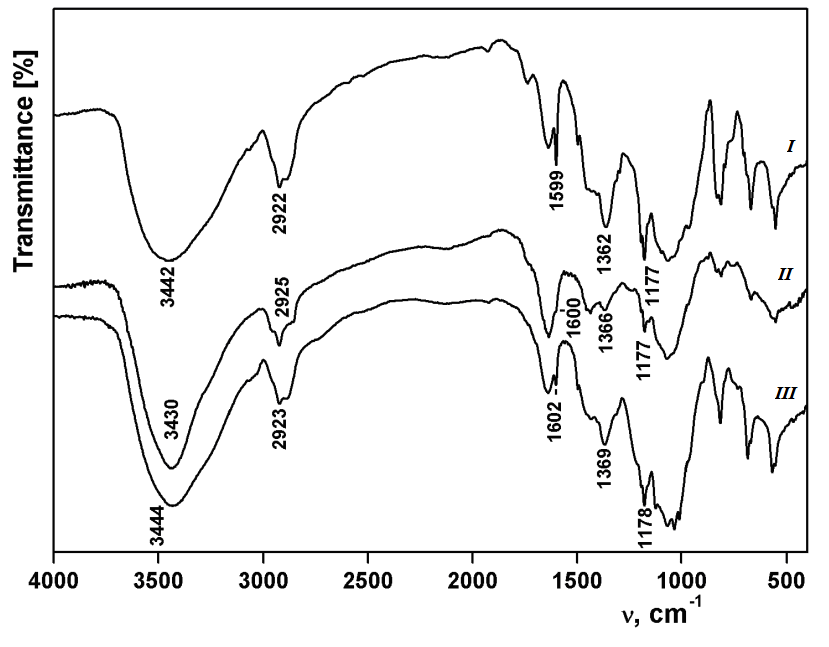
The modification of powdered ultradisperse cellulose by hindered phenol fragments was carried out. The modification was carried out by reacting cellulose tosylate with 3-(3',5'-ditert-butyl-4'-hydroxyphenyl) propionic acid hydrazide and 3,5-ditert-butyl-4-hydroxybenzyl dimethylamine in DMF at 100 ○C for 16–30 hours. Samples of modified cellulose were characterized by spectroscopy IR and 1H NMR. From the of elemental analysis data, the degree of substitution of cellulose derivatives by hindered phenol units was calculated. The antiradical activity of the obtained samples in their reactions with a stable radical 2,2-diphenyl-1-picrylhydrazyl (DPPH) was determined. The reactions of modified cellulose with DPPH were carried out under the pseudo first-order conditions with respect to the radical. From the values of the effective constants, the second-order rate constants were calculated. It was established that the modification of cellulose by hindered phenol fragments leads to a sharp increase in its antiradical activity, which depends on the degree of substitution of the sample and the method of substitution. The difference in the activity of hydrazide and benzyl derivatives of cellulose may indicate their different spatial structure, resulting in different availability of phenol fragments. The antiradical activity of the hydrazide derivative of cellulose exceeds that of the antioxidant Ionol (2,6-ditert-butyl-4-methylphenol).
Downloads
Metrics
References
Men'shchikova Ye.B., Lankin V.Z., Zenkov N.K., Bondar' I.A., Krugovykh N.F., Trufakin V.A.. Okislitel'nyy stress. Prooksidanty i antioksidanty. [Oxidative stress. Prooxidants and antioxidants]. Moscow, 2006, 554 p. (in Russ.).
Aref'yev D.V., Belostotskaya I.S., Vol'yeva V.B., Domnina N.S., Komissarova N.L., Sergeyeva O.Yu., Khrustaleva R.S. Izvestiya Akademii Nauk, seriya khimiya, 2007, no. 4, pp. 751–761. DOI: 10.1007/s11172-007-0117-x. (in Russ.).
Torlopov M.A., Chukicheva I.Yu., Kuchin A.V. Khimiya prirodnykh soyedineniy, 2011, vol. 47, no. 6, pp. 761–763. (in Russ.).
Patent 2497828 (RU). 2013. (in Russ.).
Patent 2619934 (RU). 2017. (in Russ.).
Autlov S.A., Bazarnova N.G., Kushnir Ye.Yu. Khimiya Rastitel'nogo Syr'ya, 2013, no. 3, pp. 33–41. DOI: 10.14258/jcprm.1303033. (in Russ.).
Savel'yeva Ye.Ye., Dosadina E.E., Belov A.A. Uspekhi v khimii i khimicheskoy tekhnologii, 2016, vol. 30, no. 9, pp. 16–18. (in Russ.).
Savel'yeva Ye.Ye., Dosadina E.E., Belov A.A. Uspekhi v khimii i khimicheskoy tekhnologii, 2017, vol. 31, no. 9, pp. 14–16. (in Russ.).
Patent 2554629 (RU). 2015. (in Russ.).
Qu J., Khan F. Z., Satoh M., Wada J., Hayashi H., Mizoguchi K., Masuda T. Polymer, 2008, vol. 49, pp. 1490–1496. DOI: 10.1016/j.polymer.2008.01.065.
Yang T., Xiao P., Zhang J.M, Jia R., Nawaz H., Chen Zh., Zhang J. ACS Appl. Mater. Interfaces, 2019, vol. 11, no. 4, pp. 4302–4310. DOI: 10.1021/acsami.8b15642.
Burlakova Ye.B. Rossiyskiy khimicheskiy zhurnal, 2007, vol. LI, no. 1, pp. 3–12. (in Russ.).
Patent 2343240 (RU). 2009. (in Russ.).
Kramer J.B., Boschelli D.H., Connor D.T. J. Heterocyclic. Chem., 1994, vol. 31, pp. 1439–1443. DOI: 10.1002/jhet.5570310625.
Rahn K., Diamantoglou M., Klemm D., Berghmans H., Heinze T. Angewandte Makromolekulare Chemie, 1996, vol. 238, no. 1, pp. 143–163. DOI: 10.1002/apmc.1996.052380113.
Tkacheva N.I., Morozov S.V., Grigor'yev I.A., Mognonov D.M., Kolchanov N.A. Vysokomolekulyarnyye soyedineni-ya, seriya B, 2013, vol. 55, no. 8, pp. 1086–1107. DOI: 10.7868/S0507547513070179. (in Russ.).
Shipina O.T., Garayeva M.R., Aleksandrov A.A. Vestnik KTU, 2009, no. 6, pp. 148–152. (in Russ.).
Arzamanova I.G., Logvinenko P.M., Gurevich Ya.A. Zhurnal fizicheskoy khimii, 1973, vol. 47, no. 3, pp. 707–708. (in Russ.).
Arzamanova I.G., Nayman M.I., Gurevich Ya.A. Zhurnal fizicheskoy khimii, 1979, vol. 53, no. 4, pp. 1007–1009. (in Russ.).
Copyright (c) 2020 chemistry of plant raw material

This work is licensed under a Creative Commons Attribution 4.0 International License.

This work is licensed under a Creative Commons Attribution 4.0 International License.
The authors, which are published in this journal, agree to the following conditions:
1. Authors retain the copyright to the work and transfer to the journal the right of the first publication along with the work, at the same time licensing it under the terms of the Creative Commons Attribution License, which allows others to distribute this work with the obligatory indication of the authorship of this work and a link to the original publication in this journal .
2. The authors retain the right to enter into separate, additional contractual agreements for the non-exclusive distribution of the version of the work published by this journal (for example, to place it in the university depository or to publish it in a book), with reference to the original publication in this journal.
3. Authors are allowed to post their work on the Internet (for example, in a university repository or on their personal website) before and during the review process of this journal, as this may lead to a productive discussion, as well as more links to this published work.











