STRUCTURE OF POLYPHENOLS OF LEAVES TANNING SUMAC RHUS CORIARIA L.
UDC 547.982/83/84
Abstract
The aim of this work is to study the composition and structural characteristics of the polyphenols of the tanning sumac Rhus coriaria L. of the Anacardiaceae family, growing in Uzbekistan, using a high-performance liquid chromatograph with a diode-matrix detector (HPLC-DAD) and a tandem temple mass-spectrometer (HPLC– Q-TOF-MS / MS).
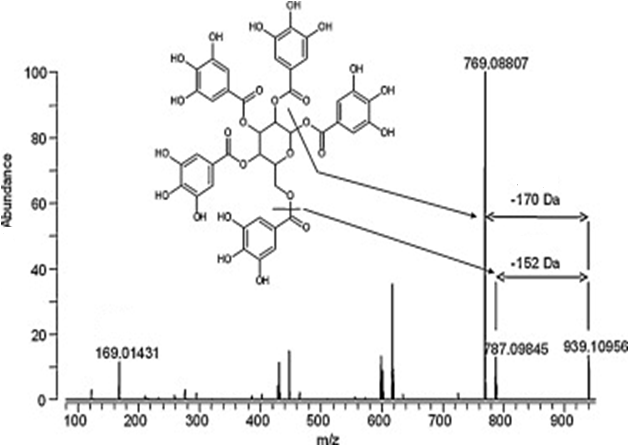
The phenolic compounds of the aerial part (leaves) of Rhus coriaria L. plants of the Anacardiaceae family were studied. For the first time, polyphenol fractions were isolated from tanning sumac leaves using stepwise hydrophobic chromatography. By HPLC, as a result of semi-preparative chromatography from the fraction eluted with 30% ethanol, 9 individual compounds were obtained, in the amount of: R-1 – 0.5 mg, R-2 – 0.8 mg, R-3 – 2.3 mg, R-4 – 12.6 mg, R-5 – 34.5 mg, R-6 – 15 mg, R-7 – 8 mg, R-8 – 7.1 mg, R-9 – 45.5 mg As a result of mass spectrometric analyzes and NMR spectroscopy for individual polyphenols, it was established that the polyphenols isolated in the individual state consist of gallic acid and glucose, interconnected by an ester bond: mono-, di-, tri-, tetra-, penta- , hexa-, hepta-, octa- and non-O-galloyl-β-D-glucose.
Downloads
Metrics
References
Ali-Shtayeh M.S., Al-Assali A.A., Jamous R.M. African Journal of Microbiology Research, 2013, no. 7, pр. 2560–2573.
Shafiei M., Nobakht M., Moazzam A.A. Pharmazie, 2011, no. 66, pр. 988–992.
Wetherilt H., Pala M. G. Charalambous, ed., Spices, Herbs and Edible Fungi: Developments in Food Science, Elsevier Science BV. Amsterdam, 1994, pp. 285–307.
Ozcan M. Journal of Medicinal Food, 2003, vol. 6, no. 1, pp. 63–66. DOI: 10.1089/109662003765184769.
Bozan B. et al. Acta Alimentaria, 2003, vol. 32, no. 1, pp. 53–61. DOI: 10.1556/AAlim.32.2003.1.7.
Candan F. Journal of Enzyme Inhibition and Medicinal Chemistry, 2003, vol. 18, no. 1, pp. 59–62. DOI: 10.1080/1475636031000069273.
Candan F., Sökmen A. Phytotherapy Research, 2004, vol. 18, no. 1, pp. 84–86. DOI: 10.1002/ptr.1228.
Pourahmad J. et al. Food and Chemical Toxicology, 2010, vol. 48, no. 3, pp. 854–858. DOI: 10.1016/j.fct.2009.12.021.
Bursal E., Köksal E. Food Research International, 2011, vol. 44, no. 7, pp. 2217–2221. DOI: 10.1016/j.foodres.2010.11.001.
Mohammadi S. et al. Journal of Pharmaceutical Sciences, 2010, vol. 18, pp. 270–275.
Giancarlo S. et al. Natural Product Research, 2006, vol. 20, no. 9, pp. 882–886. DOI: 10.1080/14786410500520186.
Lee S.H. et al. Planta Medica, 2003, vol. 69, no. 11, pp. 990–994. DOI: 10.1055/s-2003-45143.
Lee J.C. et al. Food and Chemical Toxicology, 2004, vol. 42, no. 9, pp. 1383–1388. DOI: 10.1016/j.fct.2004.03.012.
Park K.Y. et al. Journal of Ethnopharmacology, 2004, vol. 90, no. 1, pp. 73–79. DOI: 10.1016/j.jep.2003.09.043.
Panico A. et al. Journal of Medicinal Plants Research, 2009, vol. 3, pp. 855–861.
Fazeli M.O. et al. Food Control, 2007, vol. 18, no. 6, pp. 646–649. DOI: 10.1016/j.foodcont.2006.03.002.
Mohamea Khalil M.K. Journal of King Saud University, 2010, vol. 8, pp. 257–267.
Nimri L.F. et al. Pharmaceutical Biology, 1999, vol. 37, pp. 196–201. DOI: 10.1076/phbi.37.3.196.6308.
Adwan G. et al. Turkish Journal of Biology, 2010, vol. 30, pp. 239–242.
Abu-Shanab B. et al. Journal of the Islamic University of Gaza (Natural Sciences Series), 2005, vol. 13, pp. 147–153.
Gündüz G.T. et al. International Journal of Food Microbiology, 2010, vol. 141, no. 1–2, pp. 39–44. DOI: 10.1016/j.ijfoodmicro.2010.04.021.
Lin Y.M. et al. Planta Medica, 1999, vol. 65, no. 2, pp. 120–125. DOI: 10.1055/s-1999-13971.
Ahmed M.S. et al. Phytochemistry, 2001, vol. 58, no. 4, pp. 599–602. DOI: 10.1016/S0031-9422(01)00244-8.
McCutcheon A.R. et al. Journal of Ethnopharmacology, 1994, vol. 44, no. 3, pp. 157–169. DOI: 10.1016/0378-8741 (94) 01183-4.
Abu-Reidah I.M., Ali-Shtayeh M.S., Jamous R.M., Arráez-Román D., Segura-Carretero A. Food Chemistry, 2015, vol. 166, pp. 179–191.
Al-Boushi M.A., Hamdo H.H., Herbali J. International Journal of ChemTech Research, 2014, vol. 6, no. 4, pp. 2414–2420.
Kossah R., Nsabimana C., Zhang H., Chen W. Research Journal of Phytochemistry, 2010, vol. 4, pp. 146–153.
Ziyavitdinov Zh.F., Inogamov U.K., Sagdiyev N.Zh., Salikhov Sh.I. Khimiya prirodnykh soyedineniy, 1995, no. 6, pp. 862–866 (in Russ.).
Copyright (c) 2020 Khimiia rastitel'nogo syr'ia (Chemistry of plant raw material)

This work is licensed under a Creative Commons Attribution 4.0 International License.

This work is licensed under a Creative Commons Attribution 4.0 International License.
The authors, which are published in this journal, agree to the following conditions:
1. Authors retain the copyright to the work and transfer to the journal the right of the first publication along with the work, at the same time licensing it under the terms of the Creative Commons Attribution License, which allows others to distribute this work with the obligatory indication of the authorship of this work and a link to the original publication in this journal .
2. The authors retain the right to enter into separate, additional contractual agreements for the non-exclusive distribution of the version of the work published by this journal (for example, to place it in the university depository or to publish it in a book), with reference to the original publication in this journal.
3. Authors are allowed to post their work on the Internet (for example, in a university repository or on their personal website) before and during the review process of this journal, as this may lead to a productive discussion, as well as more links to this published work.











