CHEMICAL COMPOSITION AND BIOLOGICAL ACTIVITY OF SECONDARY METABOLITES FROM IMPATIENS BALSAMINA
UDC 615.322:582.776.2
Abstract
The review summarizes the literature data concerning the chemical composition of secondary metabolites and the types of biological activity of extracts and separate groups of secondary metabolites of Impatiens balsamina. First, data are given concerning the different types of biological activity of the extracts. Further, individual groups of secondary metabolites are considered, the corresponding structural formulas and types of biological activity established for this group of secondary metabolites are given. An attempt has been made to present the material about chemical composition and types of biological activity in chronological order. Extracts of I. balsamina have been shown to exhibit antiallergic, antihypotensive, antitumor, antinociceptive, antioxidant, antirheumatoid, antimicrobial, and antifungal activities. Among the secondary metabolites, peptides, naphthoquinones, polysaccharides, saponins, flavanoids, polyphenols, and tetrahydronaphthalene derivatives were identified. Research on peptides with a broad spectrum of antimicrobial activity is perspective. One of the most important groups of secondary metabolites are naphthoquinones, among which 2-methoxy-1,4-naphthoquinone is a significant metabolite, with which the antitumor effect of I. balsamina is associated. Also, this substance has shown in a number of tests an antifungal and antimicrobial activity exceeding the reference drug. Neuroprotective activity is simultaneously associated with a number of representatives of saponins, flavanoids, phenylpropanoids and tetrahydronaphthalene derivatives. This review shows that I. balsamina contains many groups of secondary metabolites, for which different types of biological activity have been identified. Due to the fact that the discussed plant is widely cultivated and is available, I. balsamina is a perspective object for the creation of new effective drugs.
Downloads
Metrics
References
Szewczyk K., Kalemba D., Komsta L. et al. Molecules, 2016, vol. 21, 1162. DOI: 10.3390/molecules21091162.
Thongnopnua P., Boonleang J., Chunejitbhong V. Analytical sciences, 1991, vol. 7, pp. 1529–1534. DOI: 10.2116/analsci.7.Supple_1529.
Srithi K., Trisonth C., Wangpakapattanawong P. et al. Journal of Ethnopharmacology, 2012, vol. 139, pp. 119–135. DOI: 10.1016/j.jep.2011.10.028.
Yincharoen K., Adekoya A.E., Chokpaisarn J. et al. Journal of Herbal Medicine, 2021, vol. 26, 100401. DOI: 10.1016/j.hermed.2020.100401.
Kang S., Goo Y., Yang M. et al. Molecules, 2013, vol. 18, pp. 6356–6365. DOI: 10.3390/molecules18066356.
Shah K.N., Verma P., Suhagia B. Journal of Applied Pharmaceutical Science, 2017, vol. 7 (08), pp. 246–252. DOI: 10.7324/JAPS.2017.70834.
Pal M., Biswas S. Molecular and Cellular Biochemistry, 1994, vol. 130, pp. 111–120. DOI: 10.1007/BF01457392.
Thevissen K., Francois I., Sijtsma L. et al. Peptides, 2005, vol. 26, pp. 1113–1119. DOI: 10.1016/j.peptides.2005.01.008.
Lucca A., Jacks T., Broekaert W. Mycopathologia, 1999, vol. 144, pp. 87–91. DOI: 10.1023/A:1007018423603.
Patel S., Osborn R., Rees S. et al. Biochemistry, 1998, vol. 37, pp. 983–990. DOI: 10.1021/bi971747d.
Tailor R., Acland D., Attenborough S. et al. The journal of biological chemistry, 1997, vol. 272, pp. 24480–24487. DOI: 10.1074/jbc.272.39.24480.
Szewczyk K., Heise E., Piwowarski J. Molecules, 2018, vol. 23, 631. DOI: 10.3390/molecules23030631.
Padwal J., Lewis W., Moody C. J. Org. Chem., 2011, vol. 76, pp. 8082–8087. DOI: 10.1021/jo201395n.
Wang Y., Li W., Wu D. et al. Evidence-Based Complementary and Alternative Medicine, 2011, vol. 2011, 704721. DOI: 10.1093/ecam/nep147.
Sakunphueak A., Panichayupakaranant P. Natural Product Research, 2012, vol. 26, pp. 1119–1124. DOI: 10.1080/14786419.2010.551297.
Panichayupakaranant P., Noguchi H., De-Eknamkul W. et al. Phytochemistry, 1995, vol. 40, no. 4, pp. 1141–1143. DOI: 10.1016/0031-9422(95)00418-7.
Ishiguro K., Ohira Y., Oku H. J. Nat. Prod., 1998, vol. 61, pp. 1126–1129. DOI: 10.1021/np9704718.
Ishiguro K., Oku H., Kato T. Phytotherapy research, 2000, vol. 14, pp. 54–56. DOI: 10.1002/(sici)1099-1573(200002)14:1<54::aid-ptr540>3.0.co;2-q.
Oku H., Ishiguro K. Biol. Pharm. Bull., 2002, vol. 25, pp. 658–660. DOI: 10.1248/bpb.25.658.
Shoji N., Umeyama A., Saitou N. et. al. Tetrahedron, 1994, vol. 50, no. 17, pp. 4973–4986. DOI: 10.1016/S0040-4020(01)90409-0.
Shoji N., Umeyama A., Saitou N. et. al. Chem. Pharm. Bull., 1994, vol. 42(7), pp. 1422–1426. DOI: 10.1248/cpb.42.1422.
Shoji N., Umeyama A., Yoshikawa K. et al. Phytochemistry, 1994, vol. 37, no. 5, pp. 1437–1441. DOI: 10.1016/s0031-9422(00)90428-x.
Li H., Yu J., Li P. Journal of Pharmaceutical and Biomedical Analysis, 2011, vol. 54, pp. 674–680. DOI: 10.1016/j.jpba.2010.10.014.
Fu Y., Gao W., Yu J. et. al. Journal of Pharmaceutical and Biomedical Analysis, 2012, vol. 64-65, pp. 64–71. DOI: 10.1016/j.jpba.2012.02.006.
Yang X., Summerhurst D., Koval S. et. al. Phytotherapy research, 2001, vol. 15, pp. 676–680. DOI: 10.1002/ptr.906.
Lee T., Suh W., Subedi L. et. al. Plants, 2020, vol. 9, 1083. DOI: 10.3390/plants9091083.
Kim D., Lee T., Subedi L. Natural Product Sciences, 2019, vol. 25(2), pp. 130–135. DOI: 10.20307/nps.2019.25.2.130.
Clevenger S. Archives of biochemistry and biophysics, 1958, vol. 76, pp. 131–138. DOI: 10.1016/0003-9861(58)90127-9.
Fukumoto H., Yamaki M., Isoi K. et al. Phytotherapy research, 1996, vol. 10, pp. 202–206. DOI: 10.1002/(SICI)1099-1573(199605)10:3<202::AID-PTR805>3.0.CO;2-0.
Fukumoto H., Ishiguro K., Murashima T. Phytochemistry, 1994, vol. 37, no. 5, pp. 1486–1488. DOI: 10.1016/s0031-9422(00)90440-0.
Hua L., Peng Z., Chia L. et al. Journal of Chromatography A, 2001, vol. 909, pp. 297–303. DOI: 10.1016/s0021-9673(00)01102-x.
Zhang Q., Cao J., Guo Z. et. al. Fitoterapia, 2015, vol. 105, pp. 234–239. DOI: 10.1016/j.fitote.2015.07.007.
Lei J., Qian S., Jiang J. Journal of Asian Natural Products Research, 2010, vol. 12, pp. 1033–1037. DOI: 10.1080/10286020.2010.532315.
Kim C., Bae M., Oh J. et al. J. Nat. Prod., 2017, vol. 80, pp. 471–478. DOI: 10.1021/acs.jnatprod.6b00981.
Panichayupakaranant P., Noguchi H., DeEknamkul W. Planta Medica, 1998, vol. 64, pp. 774–775. DOI: 10.1055/s-2006-957583.
Shin J., Ryu M., Kwon K. et al. Journal of Oral Patology & Medicine, 2015, vol. 44, pp. 420–428. DOI: 10.1111/jop.12248.
Chen X., Qian S., Feng F. Chinese Chemical Letters, 2010, vol. 21, pp. 440–442. DOI: 10.1016/j.cclet.2009.12.019.
Fukumoto H., Isoi K., Semma M. et al. Phytotherapy research, 1995, vol. 9, pp. 567–570. DOI: 10.1002/ptr.2650090806.
Ishiguro K., Fukumoto H., Murashima T. et al. Phytotherapy research, 1992, vol. 6, pp. 112–113. DOI: 10.1002/ptr.2650060213.
Ishiguro K., Fukumoto H., Osada S. et al. Phytotherapy research, 1994, vol. 8, pp. 301–304. DOI: 10.1002/ptr.2650080510.
Ishiguro K., Fukumoto H. Phytotherapy research, 1997, vol. 11, pp. 48–50. DOI: 10.1002/(SICI)1099-1573(199702)11:1<48::AID-PTR947>3.0.CO;2-3.
Oku H., Ishiguro K. Phytotherapy research, 2001, vol. 15, pp. 506–510. DOI: 10.1002/ptr.964.
Oku H., Ishiguro K. Phytotherapy research, 1999, vol. 13, pp. 521–525. DOI: 10.1002/(sici)1099-1573(199909)13:6<521::aid-ptr535>3.0.co;2-a.
Ishiguro K., Ohira Y., Oku H. Biol. Pharm. Bull., 2002, vol. 25(4), pp. 505–508. DOI: 10.1248/bpb.25.505.
Ding Z., Jiang F., Chen N. et al. Molecules, 2008, vol. 13, pp. 220–229. DOI: 10.3390/molecules13020220.
Baskar N., Parimala B., Jayakar B. IJRAP, 2012, vol. 3(4), pp. 631–633.
Shin J., Kwon K., Cho S. Pharmacognosy Magazine, 2015, vol. 11, pp. 136–142. DOI: 10.4103/0973-1296.149728.
Wang T., Cai Y., Song W. et al. BioMed Research International, 2017, 1243515. DOI: 10.1155/2017/1243515.
Mori N., Toume K., Arai M. et al. J. Nat. Med., 2011, vol. 65, pp. 234–236. DOI: 10.1007/s11418-010-0471-0.
Wang Y., Lin Y. Fitoterapia, 2012, vol. 83, pp. 1336–1344. DOI: 10.1016/j.fitote.2012.04.003.
Yew W., Kitson L., Hock A. et al. Journal of Applied Pharmaceutical Science, 2015, vol. 5(08), pp. 001–005. DOI: 10.7324/JAPS.2015.50801.
Ong J., Yong P., Lim Y. et. al. Life Sciences, 2015, vol. 135, pp. 158–164. DOI: 10.1016/j.lfs.2015.03.019.
Liew K., Yong P., Navaratnam V. et. al. Phytomedicine, 2015, vol. 22, pp. 517–527. DOI: 10.1016/j.phymed.2015.03.007.
Liew K., Yong P., Lim Y. et. al. Toxicology in Vitro, 2014, vol. 28, pp. 335–339. DOI: 10.1016/j.tiv.2013.11.008.
Daud S., Yaacob N., Fauzi A. Asian Pacific Journal of Cancer Prevention, 2021, vol. 22(S1), pp. 59–65. DOI: 10.31557/APJCP.2021.22.S1.59.
Murota H., Shinya T., Nishiuchi A. et. al. Drug. Dev. Res., 2019, vol. 80(3), pp. 395–402. DOI: 10.1002/ddr.21513.
Mansha N., Asim S., Ali H. et al. Journal of Structural Chemistry, 2020, vol. 61, pp. 182–196. DOI: 10.1134/S0022476620020031.
Imam M., Nahar N., Akter S. et al. Journal of Ethnopharmacology, 2012, vol. 140, pp. 804–810. DOI: 10.1016/j.jep.2012.06.004.
Zeng B., Chen J., Chen C. et al. Journal of Food Science, 2012, vol. 77, pp. 614–619. DOI: 10.1111/j.1750-3841.2012.02709.x.
Voravuthikunchai S.P., Kitpipit L. Clinical Microbiology and Infection, 2005, vol 11, pp. 510–512. DOI: 10.1111/j.1469-0691.2005.01104.x.
Wang Y., Wu D., Liao J. et al. The American Journal of Chinese Medicine, 2009, vol. 37, pp. 713–722. DOI: 10.1142/S0192415X09007181.
Niyomkam P., Kaewbumrung S., Kaewnpparat S. et al. Pharmaceutical Biology, 2010, vol. 48(4), pp. 375–380. DOI: 10.3109/13880200903150443.
Lee D., Shin S., Kim D. et al. Biotechnology Letters, 1999, vol. 21, pp. 1047–1050. DOI: 10.1023/A:1005636610512.
Weerden N., Bleackley M., Anderson M. Cell. Mol. Life Sci., 2013, vol. 70, pp. 3545–3570. DOI: 10.1007/s00018-013-1260-1.
Wang P., Bang J., Kim H. et al. Peptides, 2009, vol. 30, pp. 2144–2149. DOI: 10.1016/j.peptides.2009.09.020.
Fan X., Reichling J., Wink M. Pharmazie, 2013, vol. 68, pp. 628–630. DOI: 10.1691/ph.2013.6512.
Fan X., Schäfer H., Reichling J. et al. Biotechnol. J., 2013, vol. 8, pp. 1213–1220. DOI: 10.1002/biot.201300121.
Fan X., Korytowski. A., Makky A. et al. BBA – Biomembranes, 2018, vol. 1860, pp. 617–623. DOI: 10.1016/j.bbamem.2017.10.025.
Fan X., Xu W., Han J. et al. BBA – General Subjects, 2019, vol. 1863, pp. 1158–1166. DOI: 10.1016/j.bbagen.2019.04.010.
Yang X., Summerhurst D., Koval S. et. al. Phytotherapy research, 2001, vol. 15, pp. 676–680. DOI: 10.1002/ptr.906.
Jin B., Song Z., Jiang F. et al. Acta Cryst., 2011, vol. 67, o947. DOI: 10.1107/S1600536811009883.
Srivilai J., Rabgay K., Khorana N. et al. Steroids, 2016, vol. 116, pp. 67–75. DOI: 10.1016/j.steroids.2016.10.007.
Ih H., Kusharyanti I., Iwo M. International Journal of PharmTech Research, 2016, vol. 9(3), pp. 131–137.
Jiang H., Zhuang Z., Hou B. et al. Evidence-Based Complementary and Alternative Medicine, 2017, vol. 2017, 4245830. DOI: 10.1155/2017/4245830.
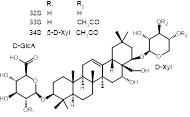
Copyright (c) 2022 chemistry of plant raw material

This work is licensed under a Creative Commons Attribution 4.0 International License.

This work is licensed under a Creative Commons Attribution 4.0 International License.
The authors, which are published in this journal, agree to the following conditions:
1. Authors retain the copyright to the work and transfer to the journal the right of the first publication along with the work, at the same time licensing it under the terms of the Creative Commons Attribution License, which allows others to distribute this work with the obligatory indication of the authorship of this work and a link to the original publication in this journal .
2. The authors retain the right to enter into separate, additional contractual agreements for the non-exclusive distribution of the version of the work published by this journal (for example, to place it in the university depository or to publish it in a book), with reference to the original publication in this journal.
3. Authors are allowed to post their work on the Internet (for example, in a university repository or on their personal website) before and during the review process of this journal, as this may lead to a productive discussion, as well as more links to this published work.











