STUDY OF THE COMPOSITION OF BIOLOGICALLY ACTIVE FORMS OF SEA BUCKTHORN (HIPPOPHAE RHAMNOIDES L.) LEAVES BY GC-MS
UDC 615.19.071
Abstract
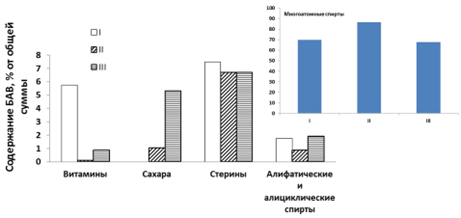
Hippophae rhamnoides L. is a perennial shrub of the Elaegnaceae family, which has a significant distribution area (both in cultivation and in the wild) and an annually renewable raw material base (fruits and leaves). The leaves of sea buckthorn are characterized by a high accumulation of various groups of biologically active substances. However, the fraction of lipophilic biologically active substances of this type of medicinal plant material remains poorly understood in terms of composition and pharmacological properties. The aim of the work was to study the phytochemical composition of the lipophilic fractions of sea buckthorn leaves by GC/MS with a predictive in silico assessment of promising types of pharmacological activity of the identified compounds for the subsequent targeted development of medicinal herbal preparations based on this medicinal plant material with a certain spectrum of action. The object of the study was the leaves of three phenological phases of plant life collected in the territory of the Voronezh region and dried by the air-shadow method to a residual moisture content of not more than 10% in 2022. On the chromatograms in the leaves harvested in different phenophases of development, the presence of peaks of about 40 compounds is observed – 20 each in the preparations removed from the leaves of phases I and III; 16 – phases II of blanks, of which 14 compounds were identified – 7, 8, and 10 in phenophases I, II, and III, respectively.The greatest number of compounds of the group of sugars, sterols, aliphatic and alicyclic alcohols was typical for the leaves of the phenological phase III – the phase of technical maturity of the fruit, which is due to the accumulation of these biologically active substances in the process of life. However, leaves already in the first phase of harvesting can be considered as a potential source of vitamins and sterols due to their significant accumulation. The results of the in silico study position phytosterols (betulin and γ-sitosterol) as the target group of biologically active substances in the lipophilic fraction of the leaves of the third phase of the harvest due to the large accumulation and the presence of a high probability of hypolipidemic, hypocholesterolemic and hepatoprotective activities. At the same time, the maximum accumulation of this fraction in the leaves during the harvesting period of fruits, the main pharmacopoeial valuable raw material of this plant, contributes to the possibility of waste-free rational use of plant resources.
Downloads
Metrics
References
Dedov I.I., Shestakova M.V., Mel'nichenko G.A., Mazurina N.V., Andreyeva Ye.N., Bondarenko I.Z., Gusova Z.R., Dzgoyeva F.Kh., Yeliseyev M.S., Yershova Ye.V., Zhuravleva M.V., Zakharchuk T.A., Isakov V.A., Klepiko-va M.V., Komshilova K.A., Krysanova V.S., Nedogoda S.V., Novikova A.M., Ostroumova O.D., Pereverzev A.P., Rozhivanov R.V., Romantsova T.I., Ruyatkina L.A., Salasyuk A.S., Sasunova A.N., Smetanina S.A., Starodubova A.V., Suplotova L.A., Tkacheva O.N., Troshina Ye.A., Khamoshina M.B., Chechel'nitskaya S.M., Shestakova Ye.A., Sheremet'yeva Ye.V. Ozhireniye i metabolism, 2021, no. 18(1), pp. 5–99. DOI: 10.14341/omet12714. (in Russ.).
Sandner G., Konig A., Wallner M., Weghuber J. Current Opinion in Food Science, 2020, vol. 34, pp. 9–20. DOI: 10.1016/j.cofs.2020.09.011.
Surbeyeva Ye.S., Sipkina N.Yu., Komova S.I., Yefremova U.A., Terninko I.I. Razrabotka i registratsiya lekarstven-nykh sredstv, 2022, no. 11(3), pp. 181–194. DOI: 10.33380/2305-2066-2022-11-3-181-194. (in Russ.).
Saggu S., Divekar H.M., Gupta V., Sawhney R.C., Banerjee P.K., Kumar R. Food Chem Toxicol., 2007, vol. 45(4), pp. 609–617. DOI: 10.1016/j.fct.2006.10.008.
Usha T., Middha S.K., Goyal A.K., Karthik M., Manoj D., Faizan S., Goyal P., Prashanth H., Pande V. The Journal of Biomedical Research, 2014, vol. 28(5), pp. 406–415. DOI: 10.7555/JBR.28.20130110.
Vijayaraghavan R., Gautam A., Kumar O., Pant S.C., Sharma M., Singh S., Kumar H.T., Singh A.K., Nivsarkar M., Kaushik M.P., Sawhney R.C., Chaurasia O.P., Prasad G.B. Indian Journal of Experimental Biology, 2006, vol. 44(10), pp. 821–831.
Padwad Y., Ganju L., Jain M., Chanda S., Karan D., Kumar Banerjee P., Chand Sawhney R. International im-munopharmacology, 2006, vol. 6(1), pp. 46–52. DOI: 10.1016/j.intimp.2005.07.015.
Tanwar H., Shweta, Singh D., Singh S.B., Ganju L. Inflammopharmacology, 2018, vol. 26(1), pp. 291–301. DOI: 10.1007/s10787-017-0345-0.
Ren Z., Gong H., Zhao A., Zhang J., Yang C., Wang P., Zhang Y. Foods, 2021, vol. 10, p. 804. DOI: 10.3390/foods10040804.
Amin M., Bilal A.M., Dhyal S., Zainab R. J. of Pharmacol. & Clin. Res., 2017, vol. 2(2), 555584. DOI: 10.19080/JPCR.2017.02.555584.
Verma H., Sharma M., Chahota R., Palial A. Vet. World, 2013, vol. 6(4), pp. 205–208. DOI: 10.5455/vetworld.2013.205-208.
Ganju L., Padwad Y., Singh R., Karan D., Chanda S., Chopra M.K., Bhatnagar P., Kashyap R., Sawhney R.C. Inter-national immunopharmacology, 2005, vol. 5(12), pp. 1675–1684. DOI: 10.1016/j.intimp.2005.03.017.
Geetha S., Sai Ram M., Singh V., Ilavazhagan G., Sawhney R.C. J. Ethnopharmacol., 2002, vol. 79(3), pp. 373–378. DOI: 10.1016/s0378-8741(01)00406-8.
Arimboor R., Kumar K.S., Arumughan C. Journal of Pharmaceutical and Biomedical Analysis, 2008, vol. 47(1), pp. 31–38. DOI: 10.1016/j.jpba.2007.11.045.
Begaa S., Messaoudi M. Biological trace element research, 2019, vol. 187(1), pp. 301–306. DOI: 10.1007/s12011-018-1365-3.
Dharam P.A., Amrit K.S., Jyoti K., Tanveer N. Indo Global Journal of Pharmaceutical Sciences, 2012, vol. 2(2), pp. 108–113.
Pop R.M., Weesepoel Y., Socaciu C., Pintea A., Vincken J.P., Gruppen H. Food Chem., 2014, vol. 147, pp. 1–9. DOI: 10.1016/j.foodchem.2013.09.083.
Tarasov A.V., Bukharinova M.A., Khamzina Ye.I. Industriya pitaniya, 2018, vol. 3, no. 2, pp. 31–38. DOI: 10.29141/2500-1922-2018-3-2-5. (in Russ.).
Murzakhmetova M.K., Utegaliyeva R.S., Aralbayeva A.N., Lesova Zh.T. Actualscience, 2015, vol. 1, no. 5(5), pp. 26–28. (in Russ.).
Karomatov I.D., Bukayev M.K. Biologiya i integrativnaya meditsina, 2018, no. 6(23), pp. 37–47. (in Russ.).
Bagirov I.M. Farmakognosticheskoye izucheniye rasteniy semeystva Lokhovyye: avtoref. dis. … kand. farm. nauk. [Pharmacognostic study of plants of the Lochaceae family: abstract. dis. ...cand. pharm. Sci.]. Moscow, 2010, 24 p. (in Russ.).
Kramarʹov S.O., Evtushenko V.V., Shadryn V.O., Holovach O.V., Kaminsʹka T.M. Aktual'naâ Infektologiâ, 2018, no. 6(2), pp. 77–82. DOI: 10.22141/2312-413x.6.2.2018.131093. (in Ukr.).
Morozov V.I. Khimiko-farmatsevticheskiy zhurnal, 2007, vol. 41, no. 8, pp. 19–21. (in Russ.).
Bortnikova V.V. Biomeditsina, 2011, no. 3, pp. 106–108. (in Russ.).
Guliyev V.B., Gul M., Yildirim A. J. Chromatogr. B, 2004, vol. 812, pp. 291–307.
Upadhyay N.K., Kumar R., Siddiqui M.S., Gupta A. Evid. Based Complement. Alternat. Med., 2011, 659705. DOI: 10.1093/ecam/nep189.
Saggu S., Divekar H.M., Gupta V., Sawhney R.C., Banerjee P.K., Kumar R. Food and Chemical Toxicology, 2007, vol. 45, pp. 609–617.
Gupta A. The International Journal of Lower Extremity Wounds, 2005, vol. 4, pp. 88–92.
Goncharova N.P., Glushenkova A.I. Chemistry of natural Compounds, 1996, vol. 32, pp. 585–586.
Dhyani D., Maikhuri R.K., Rao K.S., Kumar L., Purohit V.K., Sundriyal M., Saxena K.G. Curr. Sci., 2007, vol. 92, pp. 1148–1152.
Zeb A. J. Biol. Sci., 2004, vol. 4, pp. 687–693.
Kukina T.P., Shcherbakov D.N., Gensh K.V., Tulysheva E.A., Salnikova O.I., Grazhdannikov A.E., Kolosova E.A. Russ. J. Bioorg. Chem., 2017, vol. 43, no. 7, pp. 747–751.
Sadowska B., Budzy´nska A., Stochmal A., Zuchowski J., Rózalska B. Microb. Pathog., 2017, vol. 107, pp. 372–379.
Rafalska A., Abramowicz K., Krauze M. World Sci. News, 2017, vol. 72, pp. 123–140.
Kumar M.Y., Tirpude R.J., Maheshwari D.T., Bansal A., Misra K. Food Chem, 2013, vol. 141, pp. 3443–3450.
Guan T.T.Y., Cenkowski S., Hydamaka A. J. Food Sci., 2005, vol. 70, pp. 514–518.
Lee H.I., Kim M.S., Lee K.M., Park S.K., Seo K.I., Kim H.J., Kim M.J., Choi M.S., Lee M.K. Food Chem. Toxicol, 2011, vol. 49, pp. 2370–2376.
Maheshwari D.T., Yogendra Kumar M.S., Verma S.K., Singh V.K., Singh S.N. Food Chem. Toxic., 2011, vol. 49, pp. 2422–2428.
Michel T., Destandau E., Le Floch G., Lucchesi M.E., Elfakir C. Food Chem., 2012, vol. 131, pp. 754–760.
Tiwari S., Bala M. Phytopharmacology, 2011, vol. 1, pp. 35–48.
Upadhyay N.K., Kumar M.S.Y., Gupta A. Food Chem Toxicol., 2010, vol. 48, pp. 3443–3448.
Gradt I., Kuhn S., Morsel J., Zvaigzne G. Proc. Natl. Acad. Sci. U.S.A., 2017, vol. 3, pp. 211–216. DOI: 10.1515/prolas-2017-0035.
Zhamanbaeva G., Murzakhmetova M., Tuleukhanov S., Danilenko M. Bull. Exp. Biol. Med., 2014, vol. 158, pp. 221–224. DOI: 10.1007/s10517-014-2734-3.
Saikia M., Handique P.J. International Journal of Life Sciences Biotechnology and Pharma Research, 2013, vol. 2(1), pp. 80–91.
Górnaś P., Šnē E., Siger A., Segliņa D. Saudi J. Biol. Sci., 2016, vol. 23, pp. 512–516.
Radenkovs V., Püssa T., Juhnevica-Radenkova K., Anton D., Seglina D. Food Biosci., 2018, vol. 24, pp. 56–66.
Jain M., Ganju L., Katiyal A., Padwad Y., Mishra K.P., Chanda S., Karan D., Yogendra K.M., Sawhney R.C. Phyto-medicine, 2008, vol. 15, pp. 793–799.
Ma X., Yang W., Kallio H., Yang B. Crit. Rev. Food Sci. Nutr., 2021, vol. 62, pp. 3798–3816.
Pap N., Reshamwala D., Korpinen R., Kilpeläinen P., Fidelis M., Furtado M.M., Granato D. Food Chem. Toxicol., 2021, vol. 153, pp. 112–284.
Ma J.S., Chang W.H., Liu G.H., Zhang S., Zheng A.J., Li Y., Cai H.Y. Poult. Sci., 2015, vol. 94, pp. 2641–2649.
Singh D.N., Shukla P.K., Bhattacharyya A., Singh Y., Sirohi R. Indian J. Poult. Sci., 2019, vol. 54, pp. 257–262.
Kovaleva N.A., Trineyeva O.V., Slivkin A.I. Sorbtsionnyye i khromatograficheskiye protsessy, 2022, vol. 22, no. 3, pp. 284–298. DOI: 10.17308/sorpchrom.2022.22/9335. (in Russ.).
Isachkin A.V., Zubik I.N., Potapova A.V., Yermakov M.A. Vestnik Kurskoy gosudarstvennoy sel'skokhozyaystvennoy akademii, 2019, no. 2, pp. 64–69. (in Russ.).
Gosudarstvennaya farmakopeya Rossiyskoy Federatsii, XIV izdaniya. [State Pharmacopoeia of the Russian Federation, XIV edition]. Moscow, 2018. URL: http://femb.ru/femb/pharmacopea.php. (in Russ.).
Trineyeva O.V., Kolosova O.A., Slivkin A.I. Vestnik Voronezhskogo gosudarstvennogo universiteta. Seriya: Khimiya. Biologiya. Farmatsiya, 2022, no. 4, pp. 130–137. (in Russ.).
Verlina A.A., Buzlama A.V., Uymanova A.S., Gudkova A.A. Vestnik Voronezhskogo gosudarstvennogo universiteta. Seriya: Khimiya. Biologiya. Farmatsiya, 2021, no. 4, pp. 61–67. (in Russ.).
Sonawane P.D., Pollier J., Sayantan P., Szymanski J., Massalha H., Meital Y., Tamar U., Malitsky S., Arendt P., Pauwels L., Almekias-Siegl E., Rogachev I., Meir S., Cárdenas P.D., Masri A., Petrikov M., Schaller H., Schaf-fer A.A., Kamble A., Giril A.P., Goossens A., Aharoni A. Nat. Plants, 2016, vol. 3, pp. 1–13. DOI: 10.1038/nplants.2016.205.
Copyright (c) 2023 chemistry of plant raw material

This work is licensed under a Creative Commons Attribution 4.0 International License.

This work is licensed under a Creative Commons Attribution 4.0 International License.
The authors, which are published in this journal, agree to the following conditions:
1. Authors retain the copyright to the work and transfer to the journal the right of the first publication along with the work, at the same time licensing it under the terms of the Creative Commons Attribution License, which allows others to distribute this work with the obligatory indication of the authorship of this work and a link to the original publication in this journal .
2. The authors retain the right to enter into separate, additional contractual agreements for the non-exclusive distribution of the version of the work published by this journal (for example, to place it in the university depository or to publish it in a book), with reference to the original publication in this journal.
3. Authors are allowed to post their work on the Internet (for example, in a university repository or on their personal website) before and during the review process of this journal, as this may lead to a productive discussion, as well as more links to this published work.











