ESTERIFICATION OF BETULIN 3-ACETATE IN MELTS OF THE MALEIC AND LEVULINIC ACIDS
UDC 547.597
Abstract
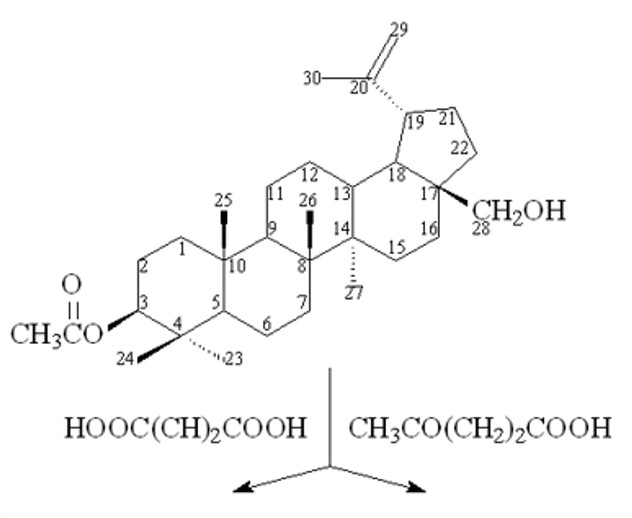
Esters of betulin containing residues of bioactive aromatic and aliphatic acids are of interest to the chemical and pharmaceutical industry as hepatoprotectors, anti-inflammatory, antiulcer and immunomodulatory substances. The development of new efficient, ecological and economical methods for the synthesis of betulin esters is an actual task. A new "green" method for the production of betulin 3-acetate-28-maleate and betulin 3-acetate-28-levulinate has been developed. For the first time, esterification of betulin 3-acetate with melts of maleic acid and levulinic acids was carried out at a temperature of 185-200°C for 5-7 minutes to obtain betulin 3-acetate-28-maleate and betulin 3-acetate-28-levulinate, respectively. The structure of the resulting betulin esters was determined using IR and NMR spectroscopy, and the composition was determined by elemental analysis. The advantage of the developed method for the synthesis of 3-acetate-28-maleate and 3-acetate-28-levulinate of betulin in comparison with the known ones is: the synthesis is carried out in the absence of harmful and hazardous solvents (pyridine, methylene chloride, chloroform), a reduction in the duration of synthesis from 15-40 hours to 5-7 minutes. Maleic anhydride is used instead of maleic anhydride on the preparation of betulin 3-acetate-28-maleate.
Downloads
Metrics
References
Tolstikov G.A., Flekhter O.B., Shul'ts E.E., Baltina L.A., Tolstikov A.G. Khimiya v interesakh ustoychivogo razvitiya, 2005, vol. 13, no. 1, pp. 1–30. (in Russ.).
Jonnalagadda S.C., Suman P., Morgan D.C., Seay N.J. Studies in Natural Products Chemistry, 2017, vol. 53, pp. 45–84. DOI: 10.1016/B978-0-444-63930-1.00002-8.
Vorob'yeva O.A., Malygina D.S., Grubova Ye.V., Mel'nikova N.B. Khimiya rastitel'nogo syr'ya, 2019, no. 4, pp. 407–430. DOI: 10.14258/jcprm.2019045419. (in Russ.).
Bildziukevich U., Özdemir Z., Wimmer Z. Molecules, 2019, vol. 24, 3546. DOI: 10.3390/molecules24193546.
Amiri S., Dastghaib S., Ahmadi M., Mehrbod P., Khadem F. Behrouj H., Aghanoori M.-R., Machaj F., Ghamsari M., Rosik J., Hudecki A., Afkhami A., Hashemi M., Los M.J., Mokarram P., Madrakian T., Ghavami S. Biotechnol. Adv., 2020, vol. 38, 107409. DOI: 10.1016/j.biotechadv.2019.06.008.
Flekhter O.B., Medvedeva N.I., Karachurina L.T., Baltina L.A., Zarudii F.S., Galin F.Z., Tolstikov G.A. Pharm. Chem. J., 2002, vol. 36, pp. 29–32. DOI: 10.1023/A:1021896722692.
Tolstikova T.G., Sorokina I.V., Tolstikov G.A., Flekhter O.B. Russ. J. Bioorg. Chem., 2006, vol. 32, pp. 37–49. DOI: 10.1134/S1068162006010031.
Chue K.-T., Chang M.-S., Ten L.N. Chem. Nat. Compd., 2011, vol. 47, pp. 583–586. DOI: 10.1007/s10600-011-0001-7.
Flekhter O.B., Boreco E.I., Nigmatullina L.R., Pavlova N.I., Medvedeva N.I., Nikolaeva S.N., Ashavina O.A., Savinova O.V., Baltina L.A., Galin F.Z., Tolstikov G.A. Pharm. Chem. J., 2004, vol. 38, pp. 355–358. DOI: 10.1023/B:PHAC.0000048431.65649.bd.
Flekhter O.B., Karachurina L.T., Nigmatullina L.R., Sapozhnikova T.A., Baltina L.A., Zarudii F.S., Galin F.Z., Spi-rikhin L.V., Tolstikov G.A., Plyasunova O.A., Pokrovskii A.G. Russ. J. Bioorg. Chem., 2002, vol. 28, pp. 494–500. DOI: 10.1023/A:1021297600187.
Flekhter O.B., Medvedeva N.I., Karachurina L.T., Baltina L.A., Galin F.Z., Zarudii F.S., Tolstikov G.A. Pharm. Chem. J., 2005, vol. 39, pp. 401–404. DOI: 10.1007/s11094-005-0167-z.
Patent 6642217 (US). 04.11.2003.
Pohjala L., Alakurtti S., Ahola T., Yli-Kauhaluoma J., Tammela P. J. Nat. Prod., 2009, vol. 72, pp. 1917–1926. DOI: 10.1021/np9003245.
Levdanskiy V.A., Levdanskiy A.V. Khimiya rastitel'nogo syr'ya, 2014, no. 1, pp. 131–137. DOI: 10.14258/jcprm.1401131. (in Russ.).
Levdanskiy V.A., Kondrasenko A.A., Levdanskiy A.V., Kuznetsov B.N. Zhurnal SFU. Khimiya, 2016, vol. 9, no. 3, pp. 337–344. DOI: 10.17516/1998-2836-2016-9-3-337-344. (in Russ.).
Levdanskiy V.A., Levdanskiy A.V., Kuznetsov B.N. Zhurnal SFU. Khimiya, 2012, vol. 5, no. 3, pp. 274–280. (in Russ.).
Levdansky V.A., Levdansky A.V., Kuznetsov B.N. Chem. Nat. Compd., 2017, vol. 53, pp. 310–311. DOI: 10.1007/s10600-017-1976-5.
Levdansky V.A., Kondrasenko A.A., Levdansky A.V., Kuznetsov B.N. Chem. Nat. Compd., 2020, vol. 56, pp. 951–952. DOI: 10.1007/s10600-020-03197-7.
Copyright (c) 2023 chemistry of plant raw material

This work is licensed under a Creative Commons Attribution 4.0 International License.

This work is licensed under a Creative Commons Attribution 4.0 International License.
The authors, which are published in this journal, agree to the following conditions:
1. Authors retain the copyright to the work and transfer to the journal the right of the first publication along with the work, at the same time licensing it under the terms of the Creative Commons Attribution License, which allows others to distribute this work with the obligatory indication of the authorship of this work and a link to the original publication in this journal .
2. The authors retain the right to enter into separate, additional contractual agreements for the non-exclusive distribution of the version of the work published by this journal (for example, to place it in the university depository or to publish it in a book), with reference to the original publication in this journal.
3. Authors are allowed to post their work on the Internet (for example, in a university repository or on their personal website) before and during the review process of this journal, as this may lead to a productive discussion, as well as more links to this published work.











