COMPARATIVE STUDY OF THE RHEOLOGICAL BEHAVIOR OF SOLUTIONS OF THE CHITIN-GLUCAN COM-PLEX FROM THE FRUIT BODIES OF ARMILLARIA MELLEA IN ACETIC AND HYDROGENIC ACID
UDC 635.89:66.061.3:532.135
Abstract
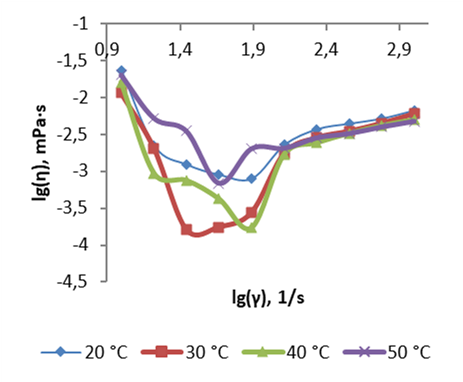
The article is devoted to the study of the rheological properties of the chitin-glucan complex (CGC) in aqueous solutions of hydrochloric and acetic acids. The CGC sample was isolated from the fruiting bodies of the Armillaria mellea, biotechnologically obtained from an easily renewable plant material. The flow of solutions of chitin-glucan complex with a concentration of 1, 5, 10% (wt.) in hydrochloric acid and 1, 3, 5% (wt.) in acetic acid was studied by the method of rotational viscometry in the range from 10 s-1 to 1000 s-1 at temperatures from 20°C to 50°C. The values of the rheological coefficients of the Ostwald equation are determined. The phenomena of non-Newtonian viscosity anomalies are established. The high viscosity of solutions may be due to the presence of an internal supramolecular structure in solutions of the chitin-glucan complex. The predominantly pseudoplastic nature of the rheological behavior of the studied solutions is shown. The flow index in this case varies from 0,18 to 0,79 for solutions of the chitin-glucan complex in hydrochloric acid and from 0,01 to 0,47 in solutions of acetic acid with a concentration of 3 to 5% (wt.). The pseudoplastic flow mechanism of such solutions can be explained by the destruction of the internal structure of the solution with an increase in shear loads. The dilatant nature of the flow of CGC solutions in acetic acid at a concentration of 1% (wt.) at a shear rate of 10 to 100 s-1 was also revealed. The flow index in this case ranges from 1,28 to 1,57. The dilatant nature of the flow may be due to the predominance of the processes of formation of a new internal structure in solution over the destruction of the existing structure in solution at a low concentration of the chitin-glucan complex. The influence of temperature on the rheological behavior of solutions is strongly distorted by the influence of other factors.
Downloads
Metrics
References
Muzzarelli R.A.A., Boudrant J., Meyer D., Manno N., Demarchis M., Paoletti M.G. Carbohydrate Polymer, 2012, vol. 87, no. 2, pp. 995−1012. DOI: 10.1016/j.carbpol.2011.09.063.
Heux L., Brugnerotto J., Desbri`ere, J., Versal, M. F., Rinaud M. Biomacromolecules, 2000, vol. 1 (4), pp. 746−751. DOI: 10.1021/bm000070y.
Ivshin V.P., Artamonova S.D., Ivshina T.N., Sharnina F.F. Vysokomolekulyarnyye soyedineniya. Seriya B, 2007, vol. 49, no. 12, pp. 2215−2222. EDN: IBMOIV. (in Russ.).
Nawawi W.M.F.B.W., Jones M., Richard J. Murphy R.J., Lee K.-Y., Kontturi E., Bismarck A. Biomacromolecules. 2020, vol. 21 (1), pp. 30−55. DOI: 10.1021/acs.biomac.9b01141.
Di Mario F., Rapana P., Tomati U., Galli E. International Journal of Biological Macromolecules, 2008, vol. 43, no. 1, pp. 8−12. DOI: 10.1016/j.ijbiomac.2007.10.005.
Zhang M., Zhao K., Zhang K., Wang W., Xing J., Li Y. Carbohydr. Polym., 2022, vol. 294. 119762. DOI: 10.1016/j.carbpol.2022.119762.
Ivshina T.N., Artamonova S.D., Ivshin V.P., Sharnina F.F. Prikladnaya biokhimiya i mikrobiologiya, 2009, vol. 45, no. 3, pp. 348−353. EDN: KAVSIH. (in Russ.).
Slivkin A.I., Belenova A.S., Shatalov G.V., Kuznetsov V.A., Slivkin D.A., Firsova L.I. Vestnik Voronezhskogo gosudarstvennogo universiteta. Seriya: Khimiya. Biologiya. Farmatsiya, 2014, no. 1, pp. 134−137. EDN: SBNFED. (in Russ.).
Zhong Y., Cai J., Zhang L.N. Chin. J. Polym. Sci., 2020, vol. 38, pp. 1047−1060. DOI: 10.1007/s10118-020-2459-x.
Roy J.C., Salaün F., Giraud S., Ferri A., Chen G., Guan J. In Tech., 2017. DOI: 10.5772/intechopen.71385.
Apryatina K.V., Khramtsova Ye.M., Sivokhin A.P., Smirnova L.A. Izvestiya Ufimskogo nauchnogo tsentra RAN, 2016, no. 3-1, pp. 12-15. EDN: WJUOCH. (in Russ.).
Malkin A.Ya. Vysokomolekulyarnyye soyedineniya. Seriya A, 2009, vol. 51, no. 1, pp. 21−36. EDN: IVSKLO. (in Russ.).
Rath A., Grisin B., Pallicity T.D., Glaser L., Guhathakurta J., Oehlsen N., Simon S., Carosella S., Middendorf P., Stegbauer L. Composites Science and Technology, 2023, vol. 235. 10995. DOI: 10.1016/j.compscitech.2023.109952.
Sampath L., Ngasotter S., Layana P., Balange A.K., Nayak B.B., Xavier K.A.M. Food Hydrocoll. Health, 2022, vol. 2. 100091. DOI: 10.1016/j.fhfh.2022.100091.
Sakoshev Z.G., Blaznov A.N. Plasticheskiye massy, 2022, no. 9-10, pp. 7−9. DOI: 10.35164/0554-2901-2022-9-10-7-9. EDN WCCKGN. (in Russ.).
Minakov D.V., Minakova A.A., Markin V.I., Bazarnova N.G., Tikhonov S.L., Yegorova Ye.YU. Khimiya ras-titel'nogo syr'ya, 2023, no. 1, pp. 313–322. DOI: 10.14258/jcprm.20230112519. (in Russ.).
Pukhnachev V.V., Frolovskaya O.A., Petrova A.G. Izvestiya vysshikh uchebnykh zavedeniy. Severo-Kavkazskiy region. Seriya: Yestestvennyye nauki, 2020, no. 2, pp. 84−93. DOI 10.18522/1026-2237-2020-2-84-93. EDN: KJNJFV. (in Russ.).
Evageliou V. Int. J. Food Sci. Technol., 2020, vol. 55, pp. 1853−1861. DOI: 10.1111/ijfs.14545.
Shipovskaya A.B., Abramov A.Y., Pyshnograi G.V., Aziz A.J.H.N. Journal of Engineering Physics and Thermophys-ics, 2016, vol. 89(3), pp. 642−651. DOI: 10.1007/s10891-016-1422-8.
Liao J., Hou B., Huang H. Carbohydr. Polym., 2022, vol. 283, pp. 119−177. DOI: 10.1016/j.carbpol.2022.119177.
Araújo D., Ferreira I.C., Torres C.A., Neves L., Freitas F. J. Chem. Technol. Biotechnol., 2020, vol. 95, pp. 1277−1289. DOI: 10.1002/jctb.6325.
Araújo D., Rodrigues T., Alves V.D., Freitas F. Polymers, 2022, vol. 14. 785. DOI: 10.3390/polym14040785.
Pushpamalar J., Meganathan P., Tan H.L., Dahlan N.A., Ooi L.-T., Neerooa B.N.H.M., Essa R.Z., Shameli K., Teow S.-Y. Gels, 2021, vol. 7 (4). 153. DOI: 10.3390/gels7040153.
Nawawi W.M.F.W., Lee K.-Y., Kontturi E., Murphy R., Bismarck A. ACS Sustainable Chemistry & Engineering, 2019, vol. 7, pp. 6492−6496. DOI: 10.1021/acssuschemeng.9b00721.
Zhang M., Zhao K., Zhang K., Wang W., Xing J., Li Y. Carbohydrate Polymers, 2022, vol. 294. 119762. DOI: 10.1016/j.carbpol.2022.119762.
Agnihotri S.A., Kulkarni V.D., Kulkarn, A.R. Aminabhavi, T.M. J. Appl. Polym. Sci., 2006, vol. 102, pp. 3255−3258. DOI: 10.1002/app.24663
Mucha M. Macromol. Chem. Phys., 1997, vol. 198, pp. 471−484. DOI: 10.1002/macp.1997.021980220.
Copyright (c) 2023 chemistry of plant raw material

This work is licensed under a Creative Commons Attribution 4.0 International License.

This work is licensed under a Creative Commons Attribution 4.0 International License.
The authors, which are published in this journal, agree to the following conditions:
1. Authors retain the copyright to the work and transfer to the journal the right of the first publication along with the work, at the same time licensing it under the terms of the Creative Commons Attribution License, which allows others to distribute this work with the obligatory indication of the authorship of this work and a link to the original publication in this journal .
2. The authors retain the right to enter into separate, additional contractual agreements for the non-exclusive distribution of the version of the work published by this journal (for example, to place it in the university depository or to publish it in a book), with reference to the original publication in this journal.
3. Authors are allowed to post their work on the Internet (for example, in a university repository or on their personal website) before and during the review process of this journal, as this may lead to a productive discussion, as well as more links to this published work.











