PROSPECTS FOR MODIFYING THE STRUCTURE OF CHITIN AND CHITOSAN OF HIGHER FUNGI TO EXPAND THE POTENTIAL OF THEIR APPLIED USE
UDC 635.8:582.28: 547.995.12
Abstract
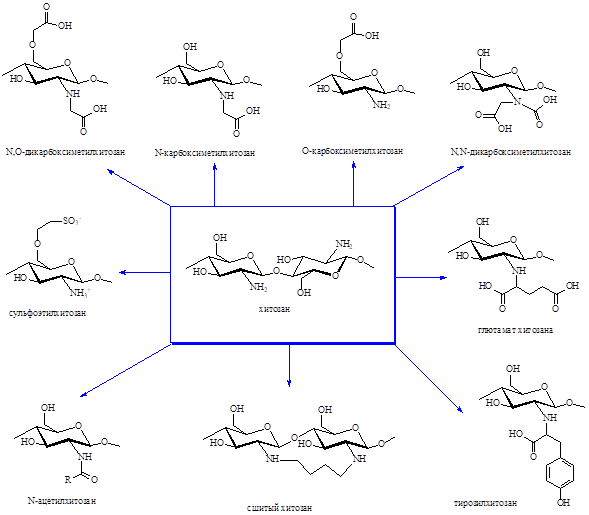
The review is devoted to summarizing scientific data in the field of the chemical structure and properties of chitin and chitosan obtained from fungal biomass, and to analyzing directions for their modification for use in medicine and the food industry as substances with antibacterial, antiviral, wound-healing and anticoagulant activity. The features of chitin biosynthesis by fungi of the Basidiomycota, Ascomycota, Deuteromycota departments and chitosan biosynthesis by fungi of the Zygomycota department are covered. It has been shown that higher fungi contain chitin in their cell walls in the form of a chitin-glucan complex, while lower fungi (zygomycetes) contain chitin in the form of chitosan-glucan. Effective components of substrates that influence the production of polysaccharides by fungi have been identified - carbohydrates in the form of glucose, sucrose and maltose, organic forms of nitrogen in the form of yeast extract and corn flour, mineral components in the form of dihydrogen phosphate and dipotassium monohydrogen phosphate. Particular attention is paid to methods for isolating chitin and modifying it to chitosan with a detailed description of the physicochemical and biological properties of polymers. The review also presents the main reactions and methods for obtaining carboxymethyl and sulfo derivatives of chitin and chitosan. The biological properties and application of these groups of substances are described. In the carboxymethylation of chitin and chitosan, the choice of appropriate reaction conditions and reagents makes it possible to obtain carboxymethyl chitin, N-, O-, N,O-carboxymethylchitosan, or N,N-dicarboxymethylchitosan. The properties and applications of carboxymethyl derivatives of chitin and chitosan strongly depend on their structure, degree of substitution, and arrangement of amino or hydroxyl groups. The main reagents in the preparation of carboxymethyl derivatives are sodium monochloroacetate, monochloroacetic and glyoxalic acids. Carboxymethyl derivatives of chitin and chitosan are used as drug delivery systems, antimicrobial agents, in tissue engineering, as components of cosmetics and food products. Modification of chitosan with sulfate groups makes it possible to obtain chitosan 2-N-, 6-O-, 2-N-6-O- and 3-O-sulphates. The main sulfonating agents are oleum, pyridine and chlorosulfonic acid. Sulfonic derivatives of chitin and chitosan can be used as a basis for obtaining hemocompatible materials (with antithrombotic and antibacterial activities).
Downloads
Metrics
References
Chang S.T. Int. J. Med. Mushrooms, 1999, vol. 1, pp. 291–301. DOI: 10.1615/INTJMEDMUSHR.V1.I4.10.
Chakravarty B. Aust. J. Agric. Eng., 2011, vol. 2, pp. 102–109.
Wani B.A., Bodha R.H., Wani A.H. J. Med. Plants Res., 2010, vol. 4, pp. 2598–2604. DOI: 10.5897/JMPR09.565.
Fraga S.M., Nunes F.M. Appl. Sci., 2020, vol. 10, article 2232. DOI: 10.3390/app10072232.
Bays H.E., Evans J.L., Maki K.C., Evans M., Maquet V., Cooper R., Anderson J.W. Eur. J. Clin. Nutr., 2013, vol. 67, pp. 2–7. DOI: 10.1038/ejcn.2012.121.
Cao Y., Zou S., Xu H., Li M., Tong Z., Xu M., Xu X. Mol. Nutr. Food Res., 2016, vol. 60, pp. 2678–2690. DOI: 10.1002/mnfr.201600032.
Wu T., Zivanovic S., Draughon F.A., Sams C.E. J Agric Food Chem., 2004, vol. 52, no. 26, pp. 7905–7910. DOI: 10.1021/jf0492565.
Giannenas I., Tsalie E., Chronis E.F., Mavridis S., Tontis D., Kyriazakis I. Anim. Feed Sci. Technol., 2011, vol. 165, pp. 218–229. DOI: 10.1016/j.anifeedsci.2011.03.002.
Synytsya A., Míˇcková K., Synytsya A., Jablonský I., Speˇvácˇek J., Erban V., Kovárˇíková E., Cˇopíková J. Carbo-hydr. Polym., 2009, vol. 76, pp. 548–556. DOI: 10.1016/j.carbpol.2008.11.021.
Xu X., Yang J., Ning Z., Zhang X. Food Funct., 2015, vol. 6, pp. 2653–2663. DOI: 10.1039/c5fo00689a.
Kalaˇc P. J. Sci. Food Agric., 2013, vol. 93, pp. 209–218. DOI: 10.1002/jsfa.5960.
Rodrigues D., Walton G., Sousa S., Rocha-Santos T.A.P., Duarte A.C., Freitas A.C., Gomes A.M.P. LWT Food Sci. Technol., 2016, vol. 73, pp. 131–139. DOI: 10.1016/J.LWT.2016.06.004.
Zhao J., Cheung P.C.K. J. Agric. Food Chem., 2011, vol. 59, pp. 5986–5992. DOI: 10.1021/jf200621y.
Carbonero E.R., Gracher A.H.P., Komura D.L., Marcon R., Freitas C.S., Baggio C.H., Santos A.R.S., Torri G., Gor-in P.A.J., Iacomini M. Food Chem., 2008, vol. 111, pp. 531–537. DOI: 10.1016/j.foodchem.2008.04.015.
Komura D.L., Carbonero E.R., Gracher A.H.P., Baggio C.H., Freitas C.S., Marcon R. Technol., 2010, vol. 101, pp. 6192–6199. DOI: 10.1016/j.biortech.2010.01.142.
Chang C.-W., Lur H.-S., Lu M.-K., Cheng J.-J. Food Res. Int., 2013, vol. 54, pp. 239–245.
Zivanovic S. Identification of opportunities for production of ingredients based on further processed fresh mushrooms, off-grade mushrooms, bi-products, and waste material. Knoxville, TN: Mushroom Council, University of Tennessee, Department of Food Science and Technology, 2006, 33 p.
Abo Elsoud M.M., El Kady E.M. Bulletin of the National Research Centre, 2019, vol. 43, article 59. DOI: 10.1186/s42269-019-0105-y.
Arrouze F., Essahli M., Rhazi M., Desbrieres J., Tolaimate A. J. Mat. Environ. Sci., 2017, vol. 8, no. 7, pp. 2251–2258.
Abdelmalek B.E., Sila A., Haddar A., Bougatef A., Ayadi M.A. Int J Biol Macromol., 2017, vol. 104, pp. 953–962. DOI: 10.1016/j.ijbiomac.2017.06.107.
Tharanathan R.N., Kittur F.S. Crit Rev Food Sci., 2003, vol. 43, pp. 61–87. DOI: 10.1080/10408690390826455.
Sitanggang A.B., Sophia L., Wu H.S. Int Food Res J., 2012, vol. 19, no. 2, pp. 393–404.
Sabir A., Altaf F., Shafiq M. Sustainable Polymer Composites and Nanocomposites., 2019, pp. 1365–1405. DOI: 10.1007/978-3-030-05399-4_46.
Cord-Landwehr S., Moerschbacher B.M. Fungal Biology and Biotechnology, 2021, vol. 8, no. 19, pp. 1–9. DOI: 10.1186/s40694 021 00127 2.
Batista A.C.L., Souza Neto F.E., Paiva W.S. Polymeros, 2018, vol. 28, no. 3, pp. 275–283. DOI: 10.1590/0104-1428.08316.
Islam S., Bhuiyan M.A.R., Islam M.N. J Polym Environ., 2017, vol. 25, pp. 854–866. DOI: 10.1007/s10924-016-0865-5.
Heux L., Brugnerotto J., Desbrieres J., Versali M.-F., Rinaudo M. Biomacromolecules, 2000, vol. 1, pp. 746–751. DOI: 10.1021/bm000070y.
Latge J.P. Mol. Microbiol., 2007, vol. 66, pp. 279–290. DOI: 10.1111/j.1365-2958.2007.05872.x.
Muzzarelli R.A. Chitin. Springer, 2011, pp. 1–34. DOI: 10.1007/978-90-481-9684-5_1.
Jones M., Kujundzic M., John S., Bismarck A. Marine Drugs, 2020, vol. 18(1), no. 64. DOI: 10.3390/md18010064.
Adams D.J. Microbiology, 2004, vol. 150(1), pp. 2029–2035. DOI: 10.1099/mic.0.26980-0.
Banks I.R., Specht C.A., Donlin M.J., Gerik K.J., Levitz S.M., Lodge J.K. Eukaryot Cell, 2005, vol. 4(11), pp. 1902–1912. DOI: 10.1128/EC.4.11.1902-1912.2005.
Baker L.G., Specht C.A., Donlin M.J., Lodge J.K. Eukaryot Cell, 2007, vol. 6(5), pp. 855–867. DOI: 10.1128/EC.00399-06.
Ruiz-Herrera J. Current topics in medical mycology. Springer, 1989, vol. 3, pp. 168–217.
Fernando L.D., Dickwella Widanage M.C., Penfield J., Lipton A.S., Washton N., Latgé J-P. Frontiers in Molecular Bi-osciences, 2021, no. 8. DOI: 10.3389/fmolb.2021.72705-3.
Akila R.M. Adv Appl Sci Res., 2014, vol. 5(4), pp. 157–170.
Gabiatti C.J., Vendruscolo F., Piaia J.C.Z., Rodrigues R.C., Durrant L.R., Costa J.A.V. Braz Arch Biol Technol., 2006, vol. 49, pp. 29–34.
Bhargav S., Panda B.P., Ali M., Javed S. Chem Biochem Eng Q, 2008, vol. 22, pp. 49–70.
Shcherba V.V., Babitskaya V.G. Prikladnaya biokhimiya i mikrobiologiya, 2008, vol. 44, no. 1, pp. 90–95. (in Russ.).
Mahapatra S., Banerjee D. Microbiology Insights, 2013, vol. 6, pp. 1–16. DOI: 10.4137/MBI.S10957.
Mishra A., Kumar S. Process Biochem., 2007, vol. 42, pp. 681–685. DOI: 10.1016/j.procbio.2006.09.022.
Nwe N., Stevens W.F. Process Biochem., 2004, vol. 39, pp. 1639–1642.
Kumirska J., Weinhold M.X., Thöming J., Stepnowski P. Polymers, 2011, vol. 3, no. 4, pp. 1875–1901. DOI: 10.3390/polym3041875.
Sudha P.N., Saranya M., Gomathi T., Gokila S., Aisverya S., Venkatesan J., Anil S. Chitosan Deriv Comp Appl., 2017, pp. 253–269. DOI: 10.1002/9781119364849.ch10.
Gbenebor O.P., Akpan E.I., Adeosun S.O. Prog Biomat., 2017, vol. 6, no. 3, pp. 97–111. DOI: 10.1007/s40204-017-0070-1.
Grishin A.A., Zorina N.V., Lutskiy V.I. Izvestiya vuzov. Prikladnaya khimiya i biotekhnologiya, 2014, no. 1(6), pp. 29–34. (in Russ.).
Younes I., Rinaudo M. Mar Drugs, 2015, vol. 13, no. 3, pp. 1133–1174. DOI: 10.3390/md13031133.
Yu Z., Lau D. Carbohyd Polym., 2017, vol. 174, pp. 941–947. DOI: 10.1016/j.carbpol.2017.06.099.
Friedman A.J., Phan J., Schairer D.O., Champer J., Qin M., Pirouz A., Modlin R.L. J Invest Dermatol., 2013, vol. 133, no. 5, pp. 1231–1239. DOI: 10.1038/jid.2012.399.
Badwan A.A., Rashid I., Al Omari M.M., Darras F.H. Mar Drugs, 2015, vol. 13, no. 3, pp. 1519–1547. DOI: 10.3390/md13031519.
Silva S.S., Mano J.F., Reis R.L. Green Chem., 2017, vol. 19, no. 5, pp. 1208–1220.
Akpan E., Gbenebor O., Adeosun S. Int J Biol Macromol., 2018, vol. 106, pp. 1080–1088. DOI: 10.1016/j.ijbiomac.2017.08.106.
Xu Q., Wang C.-H., Wayne P.D. Curr Pharm Des., 2010, vol. 16, no. 21, pp. 2350–2368. DOI: 10.2174/138161210791920469.
Nawawi W., Jones M., Murphy R.J., Lee K.-Y., Kontturi E., Bismarck A. Biomacromolecules, 2019, vol. 21, pp. 30–55. DOI: 10.1021/acs.biomac.9b01141.
Karimi K., Zamani A. Biotechnol. Adv., 2013, vol. 31, pp. 466–481. DOI: 10.1016/j.biotechadv.2013.01.009.
Jones M., Weiland K., Kujundzic M., Theiner J., Kahlig H., Kontturi E., John S., Bismarck A., Mautner A. Biomacro-molecules, 2019, vol. 20, pp. 3513–3523. DOI: 10.1021/acs.biomac.9b00791.
Hassainia A., Satha H., Boufi S. Int. J. Biol. Macromol., 2018, vol. 117, pp. 1334–1342. DOI: 10.1016/j.ijbiomac.2017.11.172.
Galbreikh L.S. Sorosovskiy obrazovatel'nyy zhurnal, 2001, vol. 7, no. 1, pp. 51–56. (in Russ.).
Nawawi W., Lee K.-Y., Kontturi E., Murphy R., Bismarck A. ACS Sustain. Chem. Eng., 2019, vol. 7, pp. 6492–6496. DOI: 10.1021/acssuschemeng.9b00721.
Araújo D., Ferreira I.C., Torres C.A.V., Neves L., Freitas F. J Chem Technol Biotechnol., 2020, vol. 95, pp. 1277–1289. DOI: 10.1002/jctb.6325.
Oliveira C.E.V., Magnani M., Sales C.V., Pontes A.L.S., Campos-Takaki G.M., Stamford T.C.M., Souza E.L. Int J Food Microbiol., 2014, vol. 171, pp. 54–61. DOI: 10.1016/j.ijfoodmicro.2013.11.006.
Moussa S.H., Tayel A.A., Al-Turki I.A. Int J Biol Macromol., 2013, vol. 54, pp. 204–208. DOI: 10.1016/j.ijbiomac.2012.12.029.
Klaykruayat B., Siralertmukul K., Srikulkit K. Carbohydr Polym., 2010, vol. 80, no. 1, pp. 197–207. DOI: 10.1016/j.carbpol.2009.11.013.
Aranaz I., Mengibar M., Harris R., Panos I., Miralles B., Acosta N., Gemma G., Heras A. Curr Chem Biol., 2009, vol. 3, no. 2, pp. 203–230. DOI: 10.2174/187231309788166415.
Lim S.H., Hudson S.M. Carbohydr Res., 2004, vol. 339, no. 2, pp. 313–319. DOI: 10.1016/j.carres.2003.10.024.
Zheng L.Y., Zhu J.F. Carbohydr Polym., 2003, vol. 54, pp. 527–530. DOI: 10.1016/j.carbpol.2003.07.009.
Pillai C., Paul W., Sharma C.P. Prog. Polym. Sci., 2009, vol. 34, pp. 641–678. DOI: 10.1016/j.progpolymsci.2009.04.001.
Alsarra I.A. Int. J. Biol. Macromol., 2009, vol. 45, pp. 16–21. DOI: 10.1016/j.ijbiomac.2009.03.010.
Hai T.A.P., Sugimoto R. Appl Surf Sci., 2018, vol. 434, pp. 188–197. DOI: 10.1016/j.apsusc.2017.10.197.
Janesch J., Jones M., Bacher M., Kontturi E., Bismarck A., Mautner A. React. Funct. Polym., 2019, vol. 146, article 104428. DOI: 10.1016/j.reactfunctpolym.2019.104428.
Xu T., Xin M., Li M., Huang H., Zhou S. Carbohydr. Polym., 2010, vol. 81, pp. 931–936. DOI: 10.1016/j.carbpol.2010.04.008.
Luan F., Wei L., Zhang J., Tan W., Chen Y., Wang P., Dong F., Li Q., Guo Z. Starch Stärke, 2018, vol. 70, no. 11–12, article 1800026. DOI: 10.1002/star.201800026.
Khattak S., Wahid F., Liu L.-P., Jia S.-R., Chu L.-Q., Xie Y.-Y., Li Z.-X., Zhong C. Appl. Microbiol. Biotechnol., 2019, vol. 103, pp. 1989–2006.
Lun'kov A.P., Il'ina A.V., Varlamov V.A. Prikladnaya biokhimiya i mikrobiologiya, 2018, vol. 54, no. 5, pp. 444–454. DOI: 10.1134/S0555109918050124. (in Russ.).
Al'myasheva N.R., Yarina M.S., Golyshkin A.V., Dzhavakhyan B.R., Krasnopol'skaya L.M. Antibiotiki i khimiotera-piya, 2017, vol. 62, no. 7–8, pp. 8–12. (in Russ.).
Yang T.-L. Int J Mol Sci., 2011, vol. 12, no. 3, pp. 1936–1963. DOI: 10.3390/ijms12031936.
Hajji S., Younes I., Ghorbel-Bellaaj O., Hajji R., Rinaudo M., Nasri M., Jellouli K. Int J Biol Macromol., 2014, vol. 65, pp. 298–306. DOI: 10.1016/j.ijbiomac.2014.01.045.
Aider M. Lebensmittel-Wissenschaft und-Technologie, 2010, vol. 43, no. 6, pp. 837–842. DOI: 10.1016/j.lwt.2010.01.021.
Kabalak M., Aracagök Y.D., Torun M. The 3rd International Symposium on EuroAsian Biodiversity. Minsk, 2017, p. 297.
Jothimani B., Sureshkumar S., Venkatachalapathy B. Carbohyd Polym., 2017, vol. 173, pp. 714–720. DOI: 10.1016/j.carbpol.2017.06.041.
Zamani A., Jeihanipour A., Edebo L., Niklasson C., Taherzadeh M.J. J Agric Food Chemistry, 2008, vol. 56, pp. 8314–8318. DOI: 10.1021/jf801478j.
Cui J., Yu Z., Lau D. Int. J. Mol. Sci., 2016, vol. 17, no. 1. DOI: 10.3390/ijms17010061.
Bamba Y., Ogawa Y., Saito T., Berglund L.A., Isogai A. Biomacromolecules, 2017, vol. 18, pp. 4405–4410. DOI: 10.1021/acs.biomac.7b01467.
Varlamov V.A., Il'ina A.V., Shagdarova B.Ts., Lun'kov A.P., Mysyakina I.S. Uspekhi biologicheskoy khimii, 2020, vol. 60, pp. 317–368. (in Russ.).
Mavisakalyan V.M., Yeritsyan K.M., Yeritsyan M.L. Problemy sovremennoy nauki i obrazovaniya, 2020, no. 8(153), pp. 12–17. (in Russ.).
Oh B.H.L., Bismarck A., Chan-Park M.B. Biomacromolecules, 2014, vol. 15, pp. 1777–1787. DOI: 10.1021/bm500172u.
Aktuganov G.E., Melentiev A.I. Applied Biochemistry and Microbiology, 2017, vol. 53, no. 6, pp. 611–627, DOI: 10.1134/S0003683817060023.
Aktuganov G.E., Melentiev A.I., Varlamov V.P. Applied Biochemistry and Microbiology, 2019, vol. 55, no. 4, pp. 323–343. DOI: 10.1134/S0003683819040021.
Minagawa T., Okamura Y., Shigemasa Y., Minami S., Okamoto Y. Carbohydr. Polym., 2007, vol. 67, pp. 640–644. DOI: 10.1016/j.carbpol.2006.07.007.
Vasconcelos D.P., Costa M., Neves N., Teixeira J.H., Vasconcelos D.M., Santos S.G. J Biomed Mat Res Part A, 2018, DOI: 10.1002/jbm.a.36370.
Cheon J.Y., Lee H.M., Park W.H. Mar Drugs, 2018, vol. 16, no. 1. DOI: 10.3390/md16010011.
Naqvi S., Moerschbacher B.M. Crit Rev Biotechnol., 2017, vol. 37, no. 1, pp. 11–25. DOI: 10.3109/07388551.2015.1104289.
Upadhyaya L., Singh J., Agarwal V., Tewari R.P. Carbohydr. Polym., 2013, vol. 91, pp. 452–466. DOI: 10.1016/j.carbpol.2012.07.076.
Liu H., Liu J., Qi C., Fang Y., Zhang L., Zhuo R., Jiang X. Acta Biomater., 2016, vol. 35, pp. 228–237. DOI: 10.1016/j.actbio.2016.02.028.
Azuma K., Nishihara M., Shimizu H., Itoh Y., Takashima O., Osaki T., Itoh N., Imagawa T., Murahata Y., Tsuka T. Biomaterials, 2015, vol. 42, pp. 20–29. DOI: 10.1016/j.biomaterials.2014.11.043.
Kim K.M., Son J.H., Kim S.K., Weller C.L., Hanna M.A. J Food Sci., 2006, vol. 71, no. 3, pp. 119–124. DOI: 10.1111/j.1365-2621.2006.tb15624.x.
Chang J., Liu W., Han B., Peng S., He B., Gu Z. Wound Repair Regen., 2013, vol. 21, pp. 113–121. DOI: 10.1111/j.1524-475X.2012.00859.x.
Anitha A., Rani V.D., Krishna R., Sreeja V., Selvamurugan N., Nair S., Tamura H., Jayakumar R. Carbohydr. Polym., 2009, vol. 78, pp. 672–677. DOI: 10.1016/j.carbpol.2009.05.028.
Mattioli-Belmonte M., Nicoli-Aldini N., De Benedittis A., Sgarbi G., Amati S., Fini M., Biagini G., Muzzarelli R. Car-bohydr. Polym., 1999, vol. 40, pp. 23–27.
Jayakumar R., Prabaharan M., Nair S.V., Tokura S., Tamura H., Selvamurugan N. Progress in Materials Science, 2010, vol. 55, no. 7, pp. 675–709. DOI: 10.1016/j.pmatsci.2010.03.001.
Patent 103288980A (CN). 11.09.2013.
Patent 2100373C1 (RU). 1996. (in Russ.).
Nudga L.A. Strukturno-khimicheskaya modifikatsiya khitina, khitozana i khitin-glyukanovykh kompleksov : dissertatsiya doktora khimicheskikh nauk. [Structural-chemical modification of chitin, chitosan and chitin-glucan complexes: disserta-tion of Doctor of Chemical Sciences]. Saint Petersburg, 2006, 360 p. (in Russ.).
Chen X.-G., Park H.-J. Carbohydrate Polymers, 2003, vol. 53, no. 4, pp. 355–359. DOI: 10.1016/s0144-8617(03)00051-1.
Fei Liu X., Lin Guan Y., Zhi Yang D., Li Z., De Yao K. Journal of Applied Polymer Science, 2000, vol. 79, no. 7, pp. 1324–1335. DOI: 10.1002/1097-4628(20010214)79:7<1324::AID-APP210>3.0.CO;2-L.
Narayanan D., Jayakumar R., Chennazhi K.P. Wiley Interdisciplinary Reviews: Nanomedicine and Nanobiotechnology. 2014, vol. 6, no. 6, pp. 574–598. DOI: 10.1002/wnan.1301.
Jones M., Kujundzic M., John S., Bismarck A. Marine Drugs, 2020, vol. 18, no. 1, article 64. DOI: 10.3390/md18010064.
Fonseca-Santos B., Chorilli M. Materials Science and Engineering, 2017, vol. 77, pp. 1349–1362. DOI: 10.1016/j.msec.2017.03.198.
Fan C., Li Z., Ji Q., Sun H., Liang Y., Yang P. Dent Mater J., 2022, vol. 41(3), pp. 392–401. DOI: 10.4012/dmj.2021-250.
Dung P., Milas M., Rinaudo M., Desbrières J. Carbohydrate Polymers, 1994, vol. 24, no. 3, pp. 209–214. DOI: 10.1016/0144-8617(94)90132-5.
Zhou H., Qian J., Wang J., Yao W., Liu C., Chen J., Cao X. Biomaterials, 2009, vol. 30, no. 9, pp. 1715–1724. DOI: 10.1016/j.biomaterials.2008.1.
Bolshakov I.N., Gornostaev L.M., Fominykh O.I., Svetlakov A.V. Polymers (Basel), 2022, vol. 14, no. 16, article 3431. DOI: 10.3390/polym14163431.
Petrova V.А., Chernyakov D.D., Moskalenko Y.E., Gasilova E.R., Strelina I.А., Okatova O.V., Baklagina Y.G., Vlasova E.N., Skorik Y.А. Carbohydr Polym., 2017, vol. 101, no. 57, pp. 866–874. DOI: 10.1016/j.carbpol.2016.10.058.
Zhang C., Ping Q., Zhang H., Shen J. Carbohyd Polym., 2003, vol. 54, no. 2, pp. 137–141. DOI: 10.1016/S0144-8617(03)00090-0.
Pires N.R., Cunha P.L.R., Maciel J.S., Angelim A.L., Melo V.M.M., de Paula R.C.M., Feitosa J.P.A. Carbohydrate Polymers, 2013, vol. 91, no. 1, pp. 92–99. DOI: 10.1016/j.carbpol.2012.08.011.
Jayakumar R., Nwe N., Tokura S., Tamura H. International Journal of Biological Macromolecules, 2007, vol. 40, no. 3, pp. 175–181. DOI: 10.1016/j.ijbiomac.2006.06.02.
Nudga L.A., Plisko E.A., Danilov S.N. Zhurnal Prikl. Khimii, 1974, no. 47, pp. 872–875.
Muzzarelli R.A.A. Carbohydrate Polymers, 1992, vol. 19, no. 4, pp. 231–236. DOI: 10.1016/0144-8617(92)90074-z.
Nud’ga L.A., Petrova V.A., Ben’kovich A.D., Petropavlovskii G.A. Russ. J. Appl. Chem., 2001, no. 74, pp. 145–148. DOI: 10.1023/A:1012776807384.
Gamzazade A., Sklyar A., Nasibov S., Sushkov I., Shashkov A., Knirel Y. Carbohydrate Polymers, 1997, vol. 34, no. 1–2, pp. 113–116. DOI: 10.1016/s0144-8617(97)00067-2.
Zhang K. et al. Carbohydr. Polym., 2011, vol. 83, no. 1, pp. 60–65.
Vikhoreva G. et al. Carbohydr. Polym., 2005, vol. 62, no. 4, pp. 327–332. DOI: 10.1016/j.carbpol.2005.05.022.
Karadeniz F. et al. Carbohydr. Polym. Elsevier Ltd, 2011, vol. 86, no. 2, pp. 666–671. DOI: 10.1016/j.carbpol.2011.05.005.
Huang R. et al. React. Funct. Polym., 2004, vol. 59, no. 1, pp. 41–51. DOI: 10.1016/j.reactfunctpolym.2003.11.014.
Imran M., Sajwan M., Alsuwayt B., Asif M. Saudi Pharmaceutical Journal, 2019, vol. 28, pp. 28–32. DOI: 10.1016/j.jsps.2019.11.003.
Cristiane R.M., Giacommo R., Marco A.D.P. J. Braz. Chem. Soc., 2003, vol. 14, pp. 797–802. DOI: 10.1590/S0103-50532003000500015.
Heise K., Hobisch M., Sacarescu L., Maver U., Hobisch J., Reichelt T., Sega M., Fischer S., Spirk S. Int. J. Nanomedi-cine, 2018, vol. 13, pp. 4881–4894. DOI: 10.2147/IJN.S172230.
Copyright (c) 2023 chemistry of plant raw material

This work is licensed under a Creative Commons Attribution 4.0 International License.

This work is licensed under a Creative Commons Attribution 4.0 International License.
The authors, which are published in this journal, agree to the following conditions:
1. Authors retain the copyright to the work and transfer to the journal the right of the first publication along with the work, at the same time licensing it under the terms of the Creative Commons Attribution License, which allows others to distribute this work with the obligatory indication of the authorship of this work and a link to the original publication in this journal .
2. The authors retain the right to enter into separate, additional contractual agreements for the non-exclusive distribution of the version of the work published by this journal (for example, to place it in the university depository or to publish it in a book), with reference to the original publication in this journal.
3. Authors are allowed to post their work on the Internet (for example, in a university repository or on their personal website) before and during the review process of this journal, as this may lead to a productive discussion, as well as more links to this published work.











