STUDY OF THE INFLUENCE OF TEMPERATURE AND pН LEVEL ON PROPETIES OF COLLOIDAL SOLUTIONS OF ZnSe AND CdSe QUANTUM DOTS IN THE SHELL OF CHITOSAN
Abstract
In this paper, we present a technique for the synthesis of colloidal quantum dots ZnSe and CdSe in an aqueous medium stabilized with a solution of chitosan at different pH values, and also studies of the properties of the resulting solutions as a function of temperature and acidity of the medium.
It has been established that as the pH is increased in the region of 4.50−5.45, the amount of light transmission of colloidal solutions of quantum dots in the shell of chitosan increases, both in the case of zinc selenide and in the case of cadmium selenide.
The effect of the medium acidity on the kinematic viscosity of the colloidal obtained solutions was studied. It has been established that the kinematic viscosity of colloidal solutions of ZnSe and CdSe quantum dots decreases with increasing pH of the medium in the region of 4.50−5.45: in both cases, there is a sharp drop in the kinematic viscosity in the region of 4.50–4.75 and a smoother decrease in the range of 4.75–5.25.
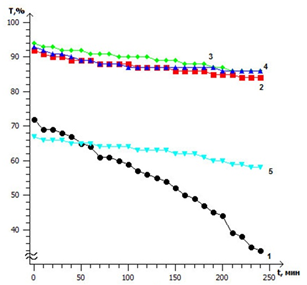
It is shown that the stabilizing effect of chitosan in colloidal solutions of semiconductor nanoparticles depends on the temperature. The most stable in time are colloidal solutions prepared at a temperature of 35, 40 and 45 °C, in which the decrease in the light transmission in time was the smallest. Solutions synthesized at temperature 25 °С, as well as at higher temperatures (35, 40, 45 и 70 °С), did not show strong aggregative stability.
Downloads
Metrics
References
Muzzarelli R.A. Chitin. Oxford, 1977. 309 р.
Stankiewicz B.A., Mastalerz M., Hof C.H.J., Bierstedt A., Flannery M.B., Briggs D.E.G., Evershed R.P. Organic Geo-chemistry, 1998, vol. 28, issue 1−2, pp. 67–76. DOI: 10.1016/S0146-6380(97)00113-7.
Takeshi H., Yoko S., Makoto O., Kousuke M. Carbohydrate Research, 2012, vol. 347, issue 1, pp. 16–22. DOI: 10.1016/j.carres.2011.09.025
Cui H., Wei W., Suayao W., Zhang L. Marine drugs, 2017, vol. 15, no. 89, pp. 212−228. DOI: 10.3390/md15040089.
Tellam R.L., Eisemann C. Insect biochemistry and molecular biology, 2000, vol. 30, issue 12, pp. 1189–1201. DOI: 10.1016/S0965-1748(00)00097-7.
Prerna D., Ramanand J. Der chemica sinica, 2012, vol. 3, no. 3, pp. 589−601.
Vincent J.V. Composites Part A: Applied Science and Manufacturing, 2002, vol. 33, issue 10, pp. 1311–1315. DOI: 10.1016/S1359-835(02)00167-7.
Ki Myong K., Jeong H.S., Sung-Koo K., Curtis L.W. Journal of Food Science, 2006, vol. 71, no. 3, pp. E119–E124. DOI: 10.1111/j.1365-2621.2006.tb15624.x
Giraud-Guille M-M., Bouligand Y. Chitin in nature and technology, 1996, vol. 6, no. 3, pp. 29–35. DOI: 10.1007/978-1-4613-2167-5_5.
Ndifor-Angwafor N.G., Anagho S.G., Nchare V. Int. J. Biol. Chem. Sci., 2013, vol. 7, no. 3.Pp. 1392−1404. DOI: 10.4314/ijbcs.v7i3.44.
Perinetti, U. Optical properties semiconductor Quantum Dots. Pisa, 2011. 144 p.
Zavideyev A.S. Molodezhnyy nauchno-tekhnicheskiy vestnik, 2012, pp. 312−319. (in Russ.).
Gerente C., Lee V. K. C., Le Cloirec P., McKay G. Crit. Rev. Environ. Sci. Technol., 2007, vol. 37, no. 1, pp. 41–127. DOI: 10/1080.10643380600729089.
Tanvi J., Sushil K., Dutta P.K. Journal of biomedical technology and research, 2015, vol. 2, no. 2, pp. 11–17. DOI: 10.19104/jbtr.2015.103.
Joyce C.C.S., Alexandra A.P., Herman S.M. Molecules, 2013, vol. 18, no. 6, pp. 6550−6572. DOI: 10.3390/molecules18066550.
Patent 2601451 (RU). 2015. (in Russ.).
Beznosyuk S.A., Shtobbe I.A., Novikova A.S. Novyye dostizheniya v khimii i tekhnologii rastitel'nogo syr'ya: materialy VI Vserossiyskoy konferentsii. [New advances in chemistry and technology of plant materials: materials of the VI All-Russian Conference]. Barnaul, 2017, pp. 111−113. (in Russ.).
Beznosyuk S.A., Shtobbe I.A., Nikiforova YA.O. Analitika Sibiri i Dal'nego Vostoka: materialy X vserossiyskoy nauchnoy konferentsii s mezhdunarodnym uchastiyem. [Analytics of Siberia and the Far East: materials of the X All-Russian Scientific Conference with international participation]. Barnaul, 2016, pp. 176–177. (in Russ.).
Kuzina L.G., Murzagil'dina A.S., Chernova V.V., Kulish Ye.I. Vestnik Bashkirskogo universiteta. Khimiya, 2012, vol. 17, no. 2, pp. 902–904.
Fedoseyeva Ye.N., Smirnova L.A., Fedoseyev V.B. Vestnik Nizhegorodskogo universiteta im. N.I. Lobachevskogo, 2008, no. 4, pp. 59–64.

This work is licensed under a Creative Commons Attribution 4.0 International License.
The authors, which are published in this journal, agree to the following conditions:
1. Authors retain the copyright to the work and transfer to the journal the right of the first publication along with the work, at the same time licensing it under the terms of the Creative Commons Attribution License, which allows others to distribute this work with the obligatory indication of the authorship of this work and a link to the original publication in this journal .
2. The authors retain the right to enter into separate, additional contractual agreements for the non-exclusive distribution of the version of the work published by this journal (for example, to place it in the university depository or to publish it in a book), with reference to the original publication in this journal.
3. Authors are allowed to post their work on the Internet (for example, in a university repository or on their personal website) before and during the review process of this journal, as this may lead to a productive discussion, as well as more links to this published work.











