BETULIN DERIVATIVES. BIOLOGICAL ACTIVITY AND SOLUBILITY IMPROVEMENT
UDC 615.322
Abstract
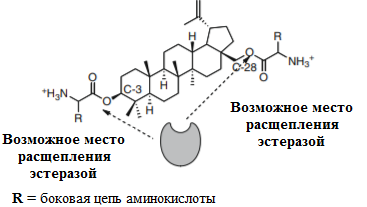
In the review the biological properties (antitumor, antiviral, hypolipidemic, anti-inflammatory, etc.) and bioavailability of betulin and betulinic acid derivatives were discussed. These compounds are isolated from various natural sources, including birch bark (Betula, Betulaceae). The structure-activity correlation was considered for well-known betulinic acid derivatives. The perspectivity of this compounds as active pharmaceutical ingredients was demonstrated by in vitro, in vivo, and ex vivo experiments. The type of antitumor actions, generally, depends on substituents at the C-3 and C-28 carbon atoms of the lupane skeleton. It is very important that the carboxyl group of betulinic acid in the C-28 position was present. In this case, the cytotoxicity of C-3 modified derivatives is extremely high for all tested cell lines.
The use of these compounds in the medical practice is complicated because they have low bioavailability and poor water solubility (from 1 to 100 µg*l-1). The main chemical syntheses for solubility improvement of betulin derivatives by grafting of hydrophilic groups were discussed. Moreover, the colloid-chemical approaches for the bioavailability improving of triterpenoids include: 1) including of these compounds in liposomes, vesicles and other nanoparticles; 2) obtaining of micelles with high-molecular compounds; 3) colloid-chemical dissolution due to physico-mechanical action; 4) inclusion complexes formation; 5) using of polymers for triterpenoids grafting. Chemical modification of betulin and betulinic acid by polar groups, such as phosphate/phosphonate, sulfate, amino acids, etc. has been shown for bioavailability improving.
Downloads
Metrics
References
Fulda S. Int J Mol Sci., 2008, no. 9, pp. 1096–1107, DOI: 10.3390/ijms9061096.
Zhang D.-M., Xu H.-G., Wang L., Li Y.-J., Sun P.-H., Wu X.-M., Wang G.-J., Chen W.-M., Ye W.-C. Medicinal Re-search Reviews, 2015, vol. 35, no. 6, p. 1127, DOI: 10.1002/med.21353.
Chudzik M., Korzonek-Szlacheta I., Król W. Molecules, 2015, no. 20, article 1610, DOI: 10.3390/molecules20011610.
Damle A.A., Pawar Y.P., Narkar A.A. Indian Journal of Experimental Biology, 2013, no. 51, p. 485.
Spivak A., Khalitova R., Nedopekina D., Dzhemileva L., Yunusbaeva M., Odinokov V., D’yakonov V., Dzhemilev U. Molecules, 2018, no. 23, article 3000, DOI: 10.3390/molecules23113000.
Woz´niak Ł., Ska’pska S., Marszałek K. Molecules, 2015, no. 20, article 20614, DOI: 10.3390/molecules201119721.
Kahnt M., Fischer L., Al-Harrasi A., Csuk R. Molecules. 2018, no. 23, article 2558, DOI: 10.3390/molecules23102558.
Drąg-Zalesińska M., Drąg M., Poręba M., Borska S., Kulbacka J., Saczko J. Cancer Cell International, 2017, no. 17, article 4, DOI: 10.1186/s12935-016-0369-3.
Sidova V., Zoufaly P., Pokorny J., Dzubak P., Hajduch M., Popa I., Urba M. PLoS ONE, 2017, no. 3, e0171621, DOI: 10.1371/journal.pone.0171621.
Król S.K., Kiełbus M., Rivero-Müller A., Stepulak A. BioMed Research International, 2015, no. 11, article 584189, DOI: 10.1155/2015/584189.
Hordyjewska A., Ostapiuk A., Horecka A. Journal of Pre-Clinical and Clinical Research, 2018, vol. 2, no. 12, pp. 72–75, DOI: 10.26444/jpccr/92743.
Boryczka S., Bębenek E., Wietrzyk J., Kempińska K., Jastrzębska M., Kusz J., Nowak M. Molecules, 2013, no. 18, ar-ticle 4526, DOI: 10.3390/molecules18044526.
Selim Y.A., Litinas K.E. Journal of the Chilean Chemical Society, 2015, vol. 60, no. 2, pp. 2896–2899, DOI: 10.4067/S0717-97072015000200007.
Zehra B., Ahmed A., Sarwar R., Khan A., Farooq U.,Ali S.A., Al-Harrasi A. Cancer Management and Research, 2019, no. 11, pp. 1667–1683, DOI: 10.2147/CMAR.S186956.
An-Qi Z., Yan Y., Yu-Qin Y., Fang-Fang Y., Mengya L., Lin-Jiang S., Ya-Li L., Yang Y.,Yu-Jue L., Yuan-Le D., Shu-Ping Y., Chen-Juan Z., Ping L., Yong-Mei X., Jin-Liang Y., Yi-Wen Z., Ting-Hong Y., Yu-Quan W. Oncotarget, 2018, vol. 9, no. 3, pp. 3794–3804, DOI: 10.18632/oncotarget.23376.
Cai Y., Zheng Y., Gu J., Wang S., Wang N., Yang B., Zhang F., Wang D., Fu W., Wang Z. Cell Death and Disease, 2018, no. 9, article 636, DOI: 10.1038/s41419-018-0669-8.
Şoica C., Dehelean C., Danciu C., Wang H., Wenz G., Ambrus R. Bojin F., Anghel M. International Journal of Mo-lecular Sciences, 2012, vol. 12, no. 13, pp. 14992–15011, DOI: 10.3390/ijms131114992.
Drag-Zalesinska M., Kulbacka J., Saczko J., Wysocka T., Zabel M., Surowiak P., Drag M. Bioorganic & Medicinal Chemistry Letters, 2009, vol. 16, no. 19, pp. 4814–4817, DOI: 10.1016/j.bmcl.2009.06.046.
Thibeault D., Gauthier C., Legault J., Bouchard J., Gagné L., Pichette A. Bioorganic & Medicinal Chemistry Letters, 2012, vol. 14, no. 22, pp. 4735–4739, DOI: 10.1016/j.bmcl.2012.05.073.
Falamaş A., Pînzaru S.C., Chiş V., Dehelean C. Journal of Molecular Structure, 2011, pp. 297–301, DOI: 10.1016/j.molstruc.2010.11.044.
Lomkova E.A., Chytil P., Janoušková O., Mueller T., Lucas H., Filippov S.K., Trhlíková O., Aleshunin P.A., Skor-ik Y.A., Ulbrich K., Etrych T. Biomacromolecules, 2016, vol. 11, no. 17, pp. 3493–3507, DOI: 10.1021/acs.biomac.6b00947.
Mullauer F.B., van Bloois L., Daalhuisen J.B., Ten Brink M.S., Storm G., Medema J.P., Schiffelers R.M., Kessler J.H. Anticancer Drugs, 2011, vol. 3, no. 22, p. 223, DOI: 10.1097/CAD.0b013e3283421035.
Saeed M.E.M., Mahmoud N., Sugimoto Y., Efferth T., Abdel-Aziz H. Frontiers in Pharmacology, 2018, no. 9, p. 481, DOI: 10.3389/fphar.2018.00481.
Liebscher G., Vanchangiri K., Mueller T., Feige K., Cavalleri J.M., Paschke R. Chemico-Biological Interactions, 2016, no. 20, p. 246 DOI: 10.1016/j.cbi.2016.01.002.
Abyshev A.Z., Abyshev R.A., Nguyen V.H., Morozova V.A. Medical Academic Journal, 2013, vol. 2, no. 13, p. 15, DOI: 10.17816/MAJ13215-32.
Periasamy G., Teketelew G., Gebrelibanos M., Sintayehu B., Gebrehiwot M., Karim A.,Geremedhin G. Archives of Applied Science Research, 2014, vol. 6, no. 3, p. 47.
Chrobak E., Bebenek E., Kadela-Tomanek M., Latocha M., Jelsch Ch., Wenger E., Boryczka S. Molecules, 2016, no. 21, article 1123, DOI: 10.3390/molecules21091123.
Zhao J., Li R., Pawlak A., Henklewska M., Sysak A., Wen L., Yi J.-E., Obmińska-Mrukowicz B. In vivo, 2018, no. 32, p. 1081, DOI: 10.21873/invivo.11349.
Pradere U., Garnier-Amblard E.C., Coats S.J., Amblard F., Schinazi R.F. Chemical Reviews, 2014, no. 114, p. 9154, DOI: 10.1021/cr5002035.
Feng J.-h., Duan X.-z., Pan J.-y., Li W.-m., Zhang X.-d., Zhang Y.-s. Tropical Journal of Pharmaceutical Research, 2017, vol. 16, no. 9, p. 2097, DOI: 10.4314/tjpr.v16i9.8.
Lee S.Y., Kim H.H., Park S.U. Experimental and Clinical Sciences, 2015, no. 14, p. 199, DOI: 10.17179/excli2015-150.
Wang Y.-J., Liu J.-B., Dou Y.-C. International Journal of Clinical and Experimental Pathology, 2015, vol. 8, no. 1, p. 252.
Boparai A., Niazi J., Bajwa N. et al. Journal of Translational Medicine, 2017, vol. 1, no. 2, p. 53, DOI: 10.15406/oajtmr.2017.01.00012.
Yi J., Xia W., Wu J., Yuan L., Wu J., Tu D., Fang J., Tan Zh. Journal of Veterinary Science, 2014, vol. 1, no. 15, p. 141, DOI: 10.4142/jvs.2014.15.1.141.
Xu T., Pang Q., Wang Y., Yan X. International journal of molecular medicine, 2017, no. 40, p. 1669, DOI: 10.3892/ijmm.2017.3163.
Cháirez-Ramírez M.H., Moreno-Jiménez M.R., González-Laredo R.F., Gallegos-Infante J.A., Rocha-Guzmán N.E. Ex-perimental and Clinical Sciences, 2016, no. 15, p. 758, DOI: 10.17179/excli2016-642.
Zhan X.K., Li J.L., Zhang S., Xing P.Y., Xia M.F. Oncology letters, 2018, no. 16, pp. 3628–3634, DOI: 10.3892/ol.2018.9097.
Zhou Z., Zhu C., Cai Z., Zhao F., He L., Lou X., Qi X. Oncology letters, 2018, no. 15, p. 7319, DOI: 10.3892/ol.2018.8183.
Alqahtani A., Hamid K., Kam A., Wong K.H., Abdelhak Z., Razmovski-Naumovski V., Chan K., Li K.M., Groundwa-ter P.W., Li G.Q. Current Medicinal Chemistry, 2013, no. 20, p. 908, DOI: 10.2174/0929867311320070007.
So H.M., Eom H.J., Lee D., Kim S., Kang K.S., Lee I.K., Baek K.H., Park J.Y., Kim K.H. Archives of Pharmacal Re-search, 2018, vol. 8, no. 41, p. 815, DOI: 10.1007/s12272-018-1064-9.
Bebenek E., Jastrzebska M., Kadela-Tomanek M., Chrobak E., Orzechowska B., Zwolinska K., Latocha M., Mertas A., Czuba Z., Boryczk S. Molecules, 2017, no. 22, article 1876, DOI: 10.3390/molecules22111876.
Härmä V., Haavikko R., Virtanen J., Ahonen I.,Schukov H.-P., Alakurtti S. et al. PLoS ONE, 2015, vol. 5, no. 10, e0126111, DOI: 10.1371/journal.pone.0126111.
Yu H., Zhang H., Chu Z., Ruan Q., Chen X., Kong D., Huang X., Li H., Tang H., Wu H., Wang Y., Xie W., Ding Y., Yao P. Oncotarget, 2017, vol. 8, no. 37, pp. 61646–61661, DOI: 10.18632/oncotarget.18661.
Haque S., Nawrot D.A., Alakurtti S., Ghemtio L., Yli-Kauhaluoma J. et al. PLoS ONE, 2014, vol. 7, no. 9, e0102696, DOI: 10.1371/journal.pone.0102696.
Shi W., Tang N., Yan W. Mini-Reviews in Organic Chemistry, 2014, no. 11, pp. 343–354, DOI: 10.2174/1570193X1103140915112124.
Bellampallia S.S., Jia Y., Moutala A., Caia S., Wijeratnec E.M.K., Gandinid M.A., Yua J., Chefdevillea A., Dora-mea A., Chewa L.A., Maduraa C.L., Luoa S., Molnara G., Khannaa M., Streichera J.M., Zamponid G.W., Gunatilakac A.L., Khanna R. Pain, 2019, no. 160, pp. 117–135, DOI: 10.1097/j.pain.0000000000001385.
Khwaza V., Oyedeji O.O., Aderibigbe B.A. Molecules, 2018, no. 23, article 2300, DOI: 10.3390/molecules23092300.
Huang Q-x., Chen H.-f., Luo X.-r., Zhang Y.-x., Yao X., Zheng X. Current Medical Science, 2018, vol. 3, no. 38, p. 387, DOI: 10.1007/s11596-018-1891-4.
Visalli R.J., Ziobrowski H., Badri K.R., He J.J., Zhang X., Arumugam S.R., Zhao H. Bioorganic & Medicinal Chemis-try Letters, 2015, vol. 16, no. 25, p. 3168, DOI: 10.1016/j.bmcl.2015.05.099.
Paduch R., Kandefer-Szerszeń M. Mini-Reviews in Organic Chemistry, 2014, no. 11, p. 262, DOI: 10.2174/1570193X1103140915105240.
Moghaddam M.G., Ahmad F.B.H., Samzadeh-Kermani A. Pharmacology & Pharmacy, 2012, no. 3, p. 119, DOI: 10.4236/pp.2012.32018.
Dang Z., Ho P., Zhu L., Qian K., Lee K.-H., Huang L., Chen C.-H. Journal of Medicinal Chemistry, 2013, vol. 5, no. 56, p. 2029, DOI: 10.1021/jm3016969.
Csuk R. Expert Opinion on Therapeutic Patents, 2014. vol. 8, no. 8, p. 913, DOI: 10.1517/13543776.2014.927441.
Yadav V.A.K. Journal of parasitic diseases, 2016, vol. 3, no. 40, p. 1082, DOI: 10.1007/s12639-014-0560-1.
Oyebanji B.O., Saba A.B., Oridupa O.A. African journal of traditional, complementary, and alternative medicines. 2014, vol. 1, no. 11, p. 30.
Haque S., Nawrot D.A., Alakurtti S., Ghemtio L., Yli-Kauhaluoma J. et al. PLoS ONE, 2014, vol. 7, no. 9, e0102696, DOI: 10.1371/journal.pone.0102696.
Zou L.-W., Dou T.-Y., Wang P., Lei W.,Weng Z.-M., Hou J., Wang D.-D., Fan Y.-M., Zhang W.-D., Ge G.-B., Yang L. Frontiers in Pharmacology, 2017, no. 8, p. 435, DOI: 10.3389/fphar.2017.00435.
Meng Q., Zhou X., Liu L., Fu S. Biomedical Journal of Scientific & Technical Research, 2018, vol. 2, no. 8, pp. 1–6, DOI: 10.26717/BJSTR.2018.08.001619.
Sousa J.L.C., Freire C.S.R., Silvestre A.J.D., Silva A.M.S. Molecules, 2019, no. 24, article 355, DOI: 10.3390/molecules24020355.
Schwiebs A., Radeke H.H. Anti-Cancer Agents in Medicinal Chemistry, 2018, no. 18, pp. 645–651, DOI: 10.2174/1871520617666171012124820.
Osunsanmi F.O., Zharare G.E., Mosa R.A., Ikhile M.I., Shode F.O., Opoku A.R. Tropical Journal of Pharmaceutical Research, 2019, vol. 2, no. 18, pp. 303–309, DOI: 10.4314/tjpr.v18i2.12.
Ríos J.L., Máñez S. Planta Med, 2018, no. 84, pp. 8–19, DOI: 10.1055/s-0043-123472.
Huang T., Chen C., Li D., Ek M. Cellulose, 2019, no. 26, pp. 665–677, DOI: 10.1007/s10570-019-02265-8.
Laavola M., Haavikko R., Hämäläinen M., Leppänen T., Nieminen R., Alakurtti S., Moreira V.M., Yli-Kauhaluoma J., Moilanen E. J Nat Prod., 2016, vol. 2, no. 79, pp. 274–280, DOI: 10.1021/acs.jnatprod.5b00709.
Wang J., Zhao Q. J Cell Biochem., 2018, pp. 1–8, DOI: 10.1002/jcb.27523.
Kim K.-S., Lee D.-S., Kim D.-Ch., Yoon Ch.-S., Ko W., Oh H., Kim Y.-Ch. Molecules, 2016, no. 21, article 1206, DOI: 10.3390/molecules21091206.
Li Y., Liu X., Jiang D., Lin Y., Wang Y., Li Q., Liu L., Hua Y. Archives of Pharmacal Research, 2016, no. 39, p. 1257, DOI: 10.1007/s12272-016-0761-5.
Oloyede H.O.B., Ajiboye H.O., Salawu M.O., Ajiboye T.O. Microbial Pathogenesis, 2017, no. 111, p. 338, DOI: 10.1016/j.micpath.2017.08.012.
Kim K.-J., Lee Y., Hwang H.-G., Sung S.H., Lee M., Son Y.-J. Journal of Clinical Medicine, 2018, vol. 6, no. 7, DOI: 10.3390/jcm7060154.
Babalola I.T., Shode F.O., Adelakun E., Opoku A.R., Mosa R.A. Journal of Pharmacognosy and Phytochemistry, 2013, vol. 1, no. 6, p. 54.
Habila A.J., Habila J.D., Shode F.O., Opoku A.R., Atawodi S.E., Umar I.A. African Journal of. Pharmacy and Phar-macology, 2013, vol. 43, no. 7, p. 2881, DOI: 10.5897/AJPP2013.3851.
Osunsanmi F.O., Zaharare G.E., Oyinloye B.E., Mosa R.A., Ikhile M.I., Shode F.O., Ogunyinka I.B., Opoku A.R. Tropical Journal of Pharmaceutical Research, 2018, vol. 10, no. 17, p. 1983, DOI: 10.4314/tjpr.v17i10.13.
Siddiqui S.A., Rahman A., Rahman M.O., Akbar M.A., Ali M.A., Al-Hemaid F.M.A., Elshikh M.S., Farah M.A. Saudi Journal of Biological Sciences, 2019, vol. 26, no. 3, pp. 554–562, DOI: 10.1016/j.sjbs.2018.03.002.
Ekuadzi E., Biney R.P., Benneh C.K., Amankwaa B.O., Jato J. Phytotherapy Research, 2018, vol. 3, no. 32, p. 480, DOI: 10.1002/ptr.5993.
Innocente A.M., Silva G.N.S., Cruz L.N., Moraes M.S., Nakabashi M., Sonnet P., Gosmann G., Garcia C.R.S., Gno-atto S.C.B. Molecules, 2012, no. 17, article 12003, DOI: 10.3390/molecules171012003.
Khan M.F., Nahar N., Rashid R.B., Chowdhury A., Rashid M.A. BMC Complementary and Alternative Medicine, 2018, vol. 48, no. 18, DOI: 10.1186/s12906-018-2116-x.
Babalola I.T., Shode F.O., Adelakun E., Opoku A.R., Mosa R.A. Journal of Pharmacognosy and Phytochemistry, 2013, vol. 1, no. 6, p. 54.
Habila A.J., Habila J.D., Shode F.O., Opoku A.R., Atawodi S.E., Umar I.A. African Journal of Pharmacy and Phar-macology, 2013, vol. 43, no. 7, p. 2881, DOI: 10.5897/AJPP2013.3851.
Osunsanmi F.O., Zaharare G.E., Oyinloye B.E., Mosa R.A., Ikhile M.I., Shode F.O., Ogunyinka I.B., Opoku A.R. Tropical Journal of Pharmaceutical Research, 2018, vol. 10, no. 17, p. 1983, DOI: 10.4314/tjpr.v17i10.13.
Schwieger-Briel A., Kiritsi D., Schempp C., Has C., Schumann H. Dermatology Research and Practice, 2017, vol. 2017, article 506896, DOI: 10.1155/2017/5068969.
Halder A., Shukla D., Das S., Roy P., Mukherjee A., Saha B. Cytokine, 2018, vol. 110, pp. 412–415.
Son L.B., Kaplun A.P., Shpilevskii A.A., Andiya-Pravdivyi Y.E., Alekseeva S.G., Gribor'ev V.B., Shvets V.I. Russ Rev Bioorg Chem., 1998, vol. 10, no. 24, pp. 700–705.
Filippov S.K., Vishnevetskaya N.S., Niebuur B.-J., Koziolová E., Lomkova E.A., Chytil P., Etrych T., Papadakis C.M. Colloid and Polymer Science, 2017, vol. 295, no. 8, p. 1313, DOI: 10.1007/s00396-017-4027-7.
Malyar Y.N., Kuznetsova S.A., Shakhtshneider T.P., Mikhailenko M.A. Journal of Siberian Federal University. Chemistry, 2015, no. 2, p. 277, DOI: 10.17516/1998-2836-2015-8-2-277-286.
Popova O.V., Sursyakova V.V., Burmakina G.V., Levdansky V.A., Rubaylo A.I. Doklady Chemistry, 2015, vol. 1, no. 461, p. 67, DOI: 10.1134/S0012500815030039.
Wang H.M., Soica C., Wenz G. Natural product communications, 2012, vol. 3, no. 7, p. 289.
Dehelean C.A., Soica C., Peev C., Ciurlea S., Feflea S., Kasa P. Farmacia, 2009, vol. 1, no. 59, p. 51.
Mikhaylenko M.A., Shakhtshneyder T.P., Drebushchak V.A., Kuznetsova S.A., Skvortsova G.P., Boldyrev V.V. Khimiya prirodnykh soyedineniy, 2011,no. 2, p. 211. (in Russ.).
Dai L., Li D., Cheng J., Liu J., Deng L.-H., Wang L.-Y., Lei J.-D., He J. Polymer Chemistry. 2014, no. 5, p. 5775, DOI: 10.1039/C4PY00648H.
Gorbunova M.N., Kraynova G.F. Vestnik permskogo nauchnogo tsentra URO RAN, 2014, no. 2, p. 44. (in Russ.).
Zawilska J.B., Wojcieszak J., Olejniczak A.B. Pharmacological Reports, 2013, vol. 65, no. 1, pp. 1–14.
Jonnalagadda S.C., Suman P., Morgan D.C., Seay J.N. In Natural Products Chemistry, 2017, vol. 53, no. 45, DOI: 10.1016/B978-0-444-63930-1.00002-8.
Tolstikov G.A., Flekhter O.B., Shul'ts E.E., Baltina JI.A., Tolstikov A.G. Khimiya v interesakh ustoychivogo razvitiya, 2005, no. 1, pp. 1–30. (in Russ.).
Kuznetsova S.A., Skvortsov G.P., Malyar YU.N., Skurydina Ye.S., Veselova O.F. Khimiya rastitel'nogo syr'ya, 2013, no. 2, pp. 93–100, DOI: 10.14258/jcprm.1302093. (in Russ.).
Flekhter O.B., Karachurina L.T., Nigmatullina L.R. Khim.-farmats. zhur., 2000, vol. 34, no. 2, pp. 3–5. (in Russ.).
Flekhter O.B., Nigmatullina L.R., Baltina L.A. Khim.-farmats. zhur., 2002, vol. 36, no. 9, pp. 26–28. (in Russ.).
Shakhtshneyder T.P., Kuznetsova S.A., Mikhaylenko M.A., Malyar Yu.N., Boldyrev V.V. Zhurnal Sibirskogo fe-deral'nogo universiteta. Khimiya, 2012, vol. 1, no. 5, pp. 52–60. (in Russ.).
Tubek B., Smuga D., Smuga M., Wawrzeńczyk C. Synthetic Communications, 2012, no. 42, p. 3648, DOI: 10.1080/00397911.2011.588817.
Tubek B., Mituła P., Niezgoda N., Kempińska K., Wietrzyk J., Wawrzeńczyk C. April Natural product communica-tions, 2013, vol. 4, no. 8, p. 435, DOI: 10.1055/s-0031-1282284.
Dai L., Cao X., Liu K.-F., Li C.-X., Zhang G.-F., Deng L.-H., Si C.-L., He J., Lei J.-D. Journal of Materials Chemis-try B, 2015, vol. 18, no. 3, pp. 3754–3766, DOI: 10.1039/c5tb00042d.
Levdanskiy V.A., Levdanskiy A.V., Kuznetsov B.N. Khimiya rastitel'nogo syr'ya, 2013, no. 1, pp. 107, DOI.org/10.14258/jcprm.1301107. (in Russ.).
Levdanskiy V.A., Levdanskiy A.V., Kuznetsov B.N. Khimiya rastitel'nogo syr'ya, 2012, no. 4, pp. 79. (in Russ.).
Bureeva S., Andia-Pravdivy J., Symon A., Bichucher A., Moskaleva V., Popenko V., Shpak A., Shvets V., Kozlov L., Kaplun A. J. Bioorganic and medicinal chemistry, 2007, vol. 15, no. 10, pp. 3489–3498, DOI: 10.1016/j.bmc.2007.03.002.
Salt A.N., Hartsock J.J., Piu F., Hou J. Audiology and Neurotology, 2018, no. 23, pp. 245–257, DOI: 10.1159/000493846.
Subbaiah M.A.M., Meanwell N.A., Kadow J.F. European Journal of Medicinal Chemistry, 2017, no. 139, p. 865, DOI: 10.1016/j.ejmech.2017.07.044.
Fukui T., Nakamura K., Sakatani T., Atsuta T., Kato T., Fukumoto T., Ito M., Inoue K., Terai A. Hinyokika Kiyo, 2017, vol. 2, no. 63, p. 57, DOI: 10.14989/ActaUrolJap_63_2_57.
Inoue T., Ogura K., Kawakita M., Tsukino H., Akamatsu S., Yamasaki T., Matsui Y., Segawa T., Sugino Y., Kamo-to T., Kamba T., Tanaka S., Ogawa O. Clinical Genitourinary Cancer, 2016, vol. 14, no. 1, e9–e17, DOI: 10.1016/j.clgc.2015.08.008.
Aurilio G., Graffeo R., Bagnardi V., Nolè F., Adamoli L., Pagani O., Gallerani E., Ferrari B., Pruneri G., Gold-hirsch A. International Journal of Clinical Oncology, 2015, vol. 1, no. 20, p. 90, DOI: 10.1007/s10147-014-0694-2.
Sorscher E.J., Hong J.S., Allan P.W., Waud W.R., Parker W.B. Cancer Chemotherapy and Pharmacology, 2012, vol. 2, no. 70, p. 321, DOI: 10.1007/s00280-012-1908-9.
Aoyama T., Hirata K., Hirata R., Yamazaki H., Yamamoto Y., Hayashi H., Matsumoto Y. Clinical Pharmacology & Therapeutics, 2012, vol. 3, no. 37, p. 356, DOI: 10.1111/j.1365-2710.2011.01297.x.
Popławska M., Borowicz K.K., Czuczwar S.J. Expert Review of Neurotherapeutics, 2015, vol. 9, no. 15, p. 983, DOI: 10.1586/14737175.2015.1074523.
Inoue Y., Usui N., Hiroki T., Shimizu K., Kobayashi S., Shimasaki S. European Journal of Drug Metabolism and Pharmacokinetics, 2013, vol. 2, no. 38, p. 139, DOI: 10.1007/s13318-012-0105-x.
Juluri A., Peddikotla P., Repka M.A., Murthy S.N. Journal of Pharmaceutical Sciences, 2013, vol. 2, no. 102, p. 500, DOI: 10.1002/jps.23373.
Patent 3966778 (US). 1974.
Patent 2936313 (US). 1958.
Patent 6689767B2 (US). 2001.
Amjad M., Carlson R. M., Krasutsky P., Karim M.R.U. Journal of Microbiology and Biotechnology. 2004, vol. 5, no. 14, pp. 1086–1088.
Patent 2539297 (RU). 2015. (in Russ.).
Copyright (c) 2019 Khimiya Rastitel'nogo Syr'ya (Chemistry of plant raw material)

This work is licensed under a Creative Commons Attribution 4.0 International License.

This work is licensed under a Creative Commons Attribution 4.0 International License.
The authors, which are published in this journal, agree to the following conditions:
1. Authors retain the copyright to the work and transfer to the journal the right of the first publication along with the work, at the same time licensing it under the terms of the Creative Commons Attribution License, which allows others to distribute this work with the obligatory indication of the authorship of this work and a link to the original publication in this journal .
2. The authors retain the right to enter into separate, additional contractual agreements for the non-exclusive distribution of the version of the work published by this journal (for example, to place it in the university depository or to publish it in a book), with reference to the original publication in this journal.
3. Authors are allowed to post their work on the Internet (for example, in a university repository or on their personal website) before and during the review process of this journal, as this may lead to a productive discussion, as well as more links to this published work.











