HYDROBORATION-OXIDATION OF GLYCIRRETHIC ACID’S DERIVATIVES
UDC 547.598.458.22
Abstract
The hydroboration-oxidation reaction is widely used in the chemistry of terpenoids both for proving the structure of new compounds isolated from natural raw materials and for the directed synthesis of low molecular weight bioregulators. Moreover, most of the known examples affect mono- and sesquiterpenes, a much smaller number - for di- and triterpenoids: most are represented by hydroboration-oxidation of localized double bonds, examples for conjugated dienes are limited only by hydroboration-oxidation of cis-eudesma-6,11-diene, abietic acid and its methyl ester.
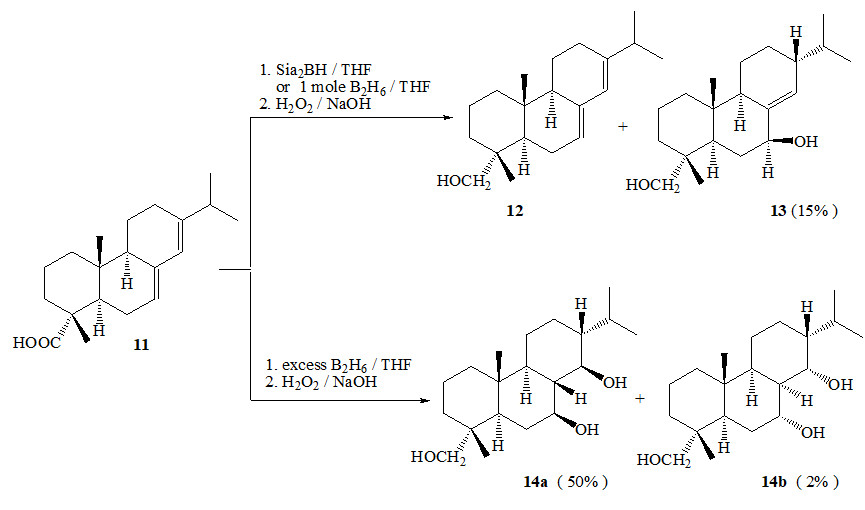
We found that the reduction of the pentacyclic triterpenoid – glycyrrhetate methyl ester – diisobutylaluminium hydride in methylene chloride at -78 ° С and subsequent hydrolysis in the presence of ammonium chloride proceeds with the formation of 3β,30-dihydroxy-18βH-olean-9(11),12(13)-diene with a yield of 90%. It was shown that the hydroboration of the 1,3-diene system in it with a 3.3 molar excess of diborane in tetrahydrofuran is accompanied by the restoration of the carboxyl function, and after oxidation with the hydrogen peroxide-sodium acetate system of the organoboranes formed, three alcohols are mixed (2 : 1 : 1): 3β,11,30-trihydroxy-18βH-olean-12(13)-ene, 3β,12,30-trihydroxy-18βH-olean-9(11)-ene and 3β,9,30-trihydroxy-18βH-olean-12 (13)-ene, respectively. A similar mixture of triols was also obtained by hydroboration-oxidation of 3β-hydroxy-18βH-olean-9(11),12(13)-diene-30-oic acid. The hydroboration-oxidation reactions of 3β,30-dihydroxy-18βH-olean-9(11),12(13)-diene or the corresponding 30th acid proceed as monoprocesses predominantly at 9(11) double bonds.
Downloads
Metrics
References
Mikhaylov B.M., Bubnov Yu.N. Bororganicheskiye soyedineniya v organicheskom sinteze. [Organoboron compounds in organic synthesis]. Moscow, 1977, 516 p. (in Russ.).
Brown H.C., Krishnamurthy S. Tetrahedro, 1979, vol. 35, no. 5, pp. 567–607. DOI: 10.1016/0040-4020(79)87003-9.
Brown H.C., Ramachandran P.V. Reduction in organic synthesis. Washington, DC, 1996, pp 1–30.
Plemenkov V.V. Vvedeniye v khimiyu prirodnykh soyedineniy. [Introduction to the chemistry of natural compounds]. Kazan, 2001, 376 p. (in Russ.).
Brown H.C., Brown H.C., Suzuki A. J. Am. Chem. Soc., 1967, vol. 89, no. 8, pp. 1933–1941. DOI: 10.1021/ja00984a031.
Acharya S.P., Brown H.C. J. Am. Chem. Soc., 1967, vol. 89, no. 8, pp. 1925–1932. DOI: 10.1021/ja00984a030.
Cocker W., Shannon P.V.R., Staniland P.A. J. Chem. Soc. C., 1967, pp. 485–489. DOI: 10.1039/J39670000485.
Macaev F.Z., Malkov A.V. Tetrahedron, 2006, vol. 62, no. 1, pp. 9–29. DOI: 10.1016/j.tet.2005.09.001.
Zweifel G., Zweifel G., Brown H.C. J. Am. Chem. Soc., 1964, vol. 86, no. 3, pp. 393–397. DOI: 10.1021/ja01057a021.
Weyerstahl P., Marschall H., Splittgerber U., Wolf D. Liebigs Ann. Chem., 1997, vol. 1997, no. 8, pp. 1783–1787. DOI: 10.1002/jlac.199719970823.
Cross B.E, Myers P.L. J. Chem. Soc. (C), 1968, pp. 471–480, DOI: 10.1039/J39680000471.
Vydrina V.A., Kravchenko A.A., Yakovleva M.P., Muslukhov R.R., Tolstikov A.G., Ishmuratov G.Yu. Khimiya prirodnykh soyedineniy, 2018, no. 3, pp. 405–407. (in Russ.); Vydrina V.A., Kravchenko А.А., Yakovleva M.P., Mus-lukhov R.R., Tolstikov A.G., Ishmuratov G.Yu. Chem. Nat. Compd., 2018, vol. 54, no. 3, pp. 478–480. DOI: 10.1007/s10600-018-2383-2 (in Russ.).
Matyukhina L.G. Zhurnal obshchey khimi, 1976, vol. 46, no. 12, pp. 2759–2760. (in Russ.).
Tolstikov G.A., Flekhter O.B., Shul'ts E.E., Baltina L.A., Tolstikov A.G. Khimiya v interesakh ustoychivogo razvitiya, 2005, no. 13, pp. 1–30.
Dračinskẏ M., Hybelbauerová S., Sejbal J., Buděšinskẏ M. Collect. Czech. Chem. Commun., 2006, vol. 71, no. 8, pp. 1131–1160. DOI: 10.1135/cccc20061131.
Klinotová E., Bosák S., Vystrčil A. Collect. Czech. Chem. Commun., 1978, vol. 43, no. 8, pp. 2204–2216. DOI: 10.1135/cccc19782204.
Inubushi Y., Sano T., Tsuda Y. Tetrahedron Lett., 1964, no. 21, pp. 1303–1310. DOI: 10.1016/S0040-4039(00)90472-6.
Vydrina V.A., Kravchenko A.A., Denisova K.S., Yakovleva M.P., Ishmuratov G.Yu. Khimiya prirodnykh soyedineniy, 2016, no. 5, p. 821. (in Russ.); Vydrina V.A., Kravchenko А.А., Denisova K.S., Yakovleva M.P., Ishmuratov G.Yu. Chem. Nat. Compd., 2016, vol. 52, no. 5, pp. 959–960. DOI: 10.1007/s10600-016-1833-y
Budayev A.S., Mikhaylova L.R., Spirikhin L.V., Baltina L.A. Khimiya prirodnykh soyedineniy, 2014, no. 2, pp. 265–267. (in Russ.); Budaev A.S., Mikhailova L.R., Spirikhin L.V., Baltina L.A. Chem. Nat. Compd., 2014, vol. 50, no. 2, pp. 302–304. DOI: 10.1007/s10600-014-0937-5.
Beseda I., Czollner L., Shah P.S., Khunt R., Gaware R., Kosma P., Stanetty C., del Ruiz-Ruiz M.C., Amer H., Mereiter K., Da Cunha T., Odermatt A., Claßen-Houben D., Jordis U. Bioorg. Med. Chem., 2010, vol. 18, no. 1, pp. 433–454. DOI: 10.1016/j.bmc.2009.10.036.
Logashenko E.B., Salomatina O.V., Markov A.V., Korchagina D.V., Salakhutdinov N.F., Tolstikov G.A., Vlassov V.V., Zenkova M.A. ChemBioChem., 2011, vol. 12, no. 5, pp. 784–794, DOI: 10.1002/cbic.201000618.
Baltina L.A., Flekhter O.B., Putiyeva ZH.M., Kondratenko R.M., Krasnova L.V., Tolstikov G.A. Khimiko-farmatsevticheskiy zhurnal, 1996, vol. 30, no. 4, pp. 47–49. (in Russ.); Baltina L.A., Flekhter O.B., Putieva Zh.M., Kondratenko R.M., Krasnova L.V., Tolstikov G.A. Pharm. Chem. J., 1996, vol. 30, no. 4, pp. 263–266. DOI: 10.1007/BF02218774.
Copyright (c) 2020 Khimiya Rastitel'nogo Syr'ya (Chemistry of plant raw material)

This work is licensed under a Creative Commons Attribution 4.0 International License.

This work is licensed under a Creative Commons Attribution 4.0 International License.
The authors, which are published in this journal, agree to the following conditions:
1. Authors retain the copyright to the work and transfer to the journal the right of the first publication along with the work, at the same time licensing it under the terms of the Creative Commons Attribution License, which allows others to distribute this work with the obligatory indication of the authorship of this work and a link to the original publication in this journal .
2. The authors retain the right to enter into separate, additional contractual agreements for the non-exclusive distribution of the version of the work published by this journal (for example, to place it in the university depository or to publish it in a book), with reference to the original publication in this journal.
3. Authors are allowed to post their work on the Internet (for example, in a university repository or on their personal website) before and during the review process of this journal, as this may lead to a productive discussion, as well as more links to this published work.











