REMOVAL OF Bi(III) IONS BY PHYTIC ACID DERIVATIVES FROM RICE BRAN
UDC 542.06:661.887:664.782.86
Abstract
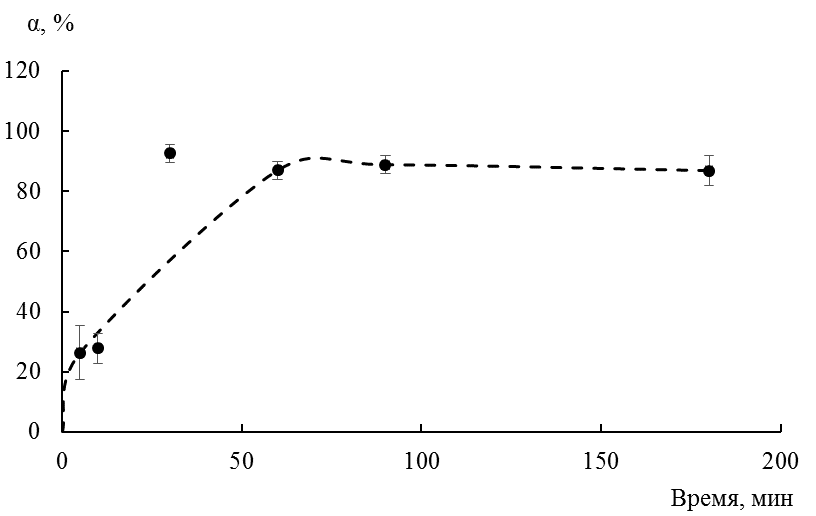
Creation of new multifunctional materials based on renewable raw materials is a major direction in recent years. Large-tonnage waste of rice production (husk, straw, bran) of the Far East is a promising raw material base for obtaining such materials. Composition of rice bran includes inositol hexaphosphoric acid and its derivatives (phytin, phosphoinositol) which are capable of chelating polyvalent metal ions. Bismuth (III) is one of natural water pollutants that come from leaching of bismuth-containing minerals and activities of pharmaceutical and perfume industries. The goal of this work is to study removal conditions of bismuth (III) ions from aqueous solutions of a phytic acid derivative obtained from rice bran. It is shown in the work that with a sorbent: solution ratio of 1: 100, bismuth ions are removed from the solution by 89 %. It was found that removal of bismuth cations depends on the initial concentration (3.17–51.90 mg/l) and varies from 13 to 96 %. A comparative analysis also showed that chromium (III) ions are removed from aqueous solutions by a phosphorus-containing product better than bismuth (III) ions. These studies allow us to give recommendations on the choice of materials for treating solutions from heavy metal ions, expanding the range of currently used natural sorbents based on plant materials and solving at the same time an urgent environmental and economic problem - the disposal of rice production wastes.
Downloads
Metrics
References
Yang N., Sun H. Encyclopedia of Environmental Health, 2011, vol. 1, pp. 414–420. DOI: 10.1016/B978-0-444-52272-6.00374-3.
Klyuchnikov A.S., Glyzina T.S., Gorchakov E.V. Khimiya i khimicheskaya tekhnologiya v XXI veke: materialy XVI Mezhdunarodnoy nauchno-prakticheskoy konferentsii studentov i molodykh uchenykh, posvyashchennoy 115-letiyu so dnya rozhdeniya professora L.P. Kulova. [Chemistry and chemical technology in the XXI century: materials of the XVI International scientific-practical conference of students and young scientists dedicated to the 115th anniversary of the birth of Professor L.P. Kuleva]. Tomsk, 2015, pp. 158–159. (in Russ.).
Gigiyenicheskiye normativy GN 2.1.5.1315-03. Predel'no dopustimyye kontsentratsii khimicheskikh veshchestv v vode vodnykh ob"yektov khozyaystvenno-pit'yevogo i kul'turno-bytovogo vodopol'zovaniya. [Hygienic standards GN 2.1.5.1315-03. Maximum permissible concentration of chemical substances in water of water bodies for household and drinking and cultural and household water use]. Moscow, 2003, 94 p. (in Russ.).
Aljerf L. Journal of Environmental Management, 2018, vol. 255, pp. 120–132. DOI: 10.1016/j.jenvman.2018.07.048.
Shashkova I.L., Rat'ko A.I., Panasygin A.S., Mil'vit N.V. and Bondareva N.V. Russian Journal of Applied Chemistry, 2001, vol. 74, no. 2, pp. 253–258. DOI: 10.1023/A:1012778202357.
Sdiri A. Environmental Progress & Sustainable Energy, 2018, vol. 37, no. 6, pp. 2034–2041. DOI: 10.1002/ep.12893.
Andreyeva N.P. Primeneniye kompleksnykh sorbentov dlya ochistki stochnykh vod ot krupnomolekulyarnykh organich-eskikh soyedineniy i ionov tyazhelykh metallov: dissertatsiya kandidata tekhnicheskikh nauk. [Application of complex sorbents for wastewater treatment from large-molecular organic compounds and heavy metal ions: Ph.D. thesis]. Mos-cow, 2006, 155 p. (in Russ.).
Makarenko N.V., Zemnukhova L.A., Nemtarev A.V., Kovekhova A.V., Arefieva O.D. BioResources, 2018, vol. 13, no. 2, pp. 3411–3419. DOI: 10.15376/biores.13.2.3411-3419.
Sidorova M.V. Razrabotka i issledovaniye kompleksov fitinovoy kisloty s biologicheski aktivnymi aminami kak kompo-nentov gidrofil'nykh geley: dissertatsiya kandidat farmatsevticheskikh nauk. [Development and research of complexes of phytic acid with biologically active amines as components of hydrophilic gels: dissertation candidate of pharmaceutical sciences]. Nizhny Novgorod, 2015, 166 p. (in Russ.).
Kel'ner R. Analiticheskaya khimiya. Problemy i podkhody. [Analytical chemistry. Problems and approaches]. Moscow, 2004, vol. 2, 728 p. (in Russ.).
Bretti C., Cigala R.M., Stefano C.D., Lando G., Sammartano S. Journal of Chemical & Engineering Data, 2012, no. 57, pp. 2838−2847. DOI: 10.1021/je300755y.
Torres J., Dominguez S., Cerda M.F., Obal G., Mederos A., Irvine R.F., Diaz A., Kremer C. Journal of Inorganic Bio-chemistry, 2005, vol. 3, no. 99, pp. 828–840. DOI: 10.1016/j.jinorgbio.2004.12.011.
Crea P., Robertis A.D., Stefano C.D., Sammartano S. Biophysical Chemistry, 2006. no. 24, pp. 18–26. DOI: 10.1016/j.bpc.2006.05.027.
Maddlach V.T., Kurnick A.A., Reld B.L. Proceedings of the Society for Experimental Biology and Medicine, 1964, no. 115, pp. 391–402.
Persson H., Türk M., Nyman M., Sandberg A.S. Journal of Agriculture and Food Chemistry, 1998, no. 46, pp. 3194–3200. DOI: 10.1021/jf971055w.
Sala M., Makuc D., Kolar J., Plavec J., Pihlar B. Carbohydrate Research, 2011, no. 346, pp. 488–494. DOI: 10.1016/j.carres.2010.12.021.
Yarusova S.B., Makarenko N.V., Gordiyenko P.S., Karpenko M.A., Novikova Ye.S. Zhurnal fizicheskoy khimii, 2018, vol. 92, no. 3, pp. 451–456. DOI: 10.1134/S0036024418030354. (in Russ.).
Kovekhova A.V., Aref'yeva O.D., Makarenko N.V., Zemnukhova L.A., Kovaleva Ye.V. Khimiya rastitel'nogo syr'ya, 2018, no. 4 pp. 281–288. DOI: 10.14258/jcprm.2018043761. (in Russ.).
Makarenko N.V., Arefieva O.D., Kovekhova A.V., Zemnukhova L.A. BioResources, 2019, vol. 14, no. 2, pp. 4866–4872. DOI: 10.15376/biores.14.2.4866-4872.
Kolzunova L.G., Zemnukhova L.A., Fedorishcheva G.A., Kurilenko L.N., Sergiyenko V.I. Zhurnal prikladnoy khimii, 2000, vol. 73, no. 10, pp. 1644–1651. (in Russ.).
Makarenko N.V., Kharchenko U.V., Slobodyuk A.B., Zemnukhova L.A. Khimiya rastitel'nogo syr'ya, 2013, no. 3, pp. 255–260. DOI: 10.14258/jcprm.1303255. (in Russ.).
Yukhin YU.M., Mikhaylov YU.I. Khimiya vismutovykh soyedineniy i materialov. [Chemistry of bismuth compounds and materials]. Novosibirsk, 2001, 360 p. (in Russ.).
Busev A.I. Analiticheskaya khimii vismuta. [Analytical chemistry of bismuth]. 1953, 382 p. (in Russ.).
Lavrukhina A.K., Yukina L.V. Analiticheskaya khimiya khroma. [Analytical chemistry of chromium]. Moscow, 1979, 227 p. (in Russ.).
Copyright (c) 2021 chemistry of plant raw material

This work is licensed under a Creative Commons Attribution 4.0 International License.

This work is licensed under a Creative Commons Attribution 4.0 International License.
The authors, which are published in this journal, agree to the following conditions:
1. Authors retain the copyright to the work and transfer to the journal the right of the first publication along with the work, at the same time licensing it under the terms of the Creative Commons Attribution License, which allows others to distribute this work with the obligatory indication of the authorship of this work and a link to the original publication in this journal .
2. The authors retain the right to enter into separate, additional contractual agreements for the non-exclusive distribution of the version of the work published by this journal (for example, to place it in the university depository or to publish it in a book), with reference to the original publication in this journal.
3. Authors are allowed to post their work on the Internet (for example, in a university repository or on their personal website) before and during the review process of this journal, as this may lead to a productive discussion, as well as more links to this published work.











