OBTAINING SUPRAMOLECULAR COMPLEXES OF ANGIDROLAGOQUILINE WITH GLYCYRRHISINOIC ACID AND ITS SALTS AND STUDYING THEIR SPECIFIC HEMOSTATIC ACTIVITY
UDC 577.1:577.352.34
Abstract
The hemostatic effect of drugs of the genus Lagochilus (Lamiaceae) is due to the content in its composition of four atomic alcohol diterpenoid lagochiline, alkaloid stahydrine, vitamin K and tannins. On the basis of lagochilin, a number of acetyl and isopropylidene derivatives were obtained. It has been shown that the hemostatic activity in a certain way depends on the number of free hydroxyl groups of lagochilin and its derivatives. However, lagochiline derivatives are poorly soluble in water, which limits their bioavailability. Chemical modification of lagochilin is carried out mainly by hydroxyl groups with the production of esters or succinates with dibasic anhydrides.
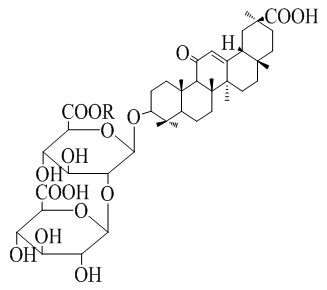
For the first time, water-soluble supramolecular complexes based on the diterpenoid lactone an-hydrolagoquiline with the triterpene saponin glycyrrhizic acid (GA) and its monoammonium (MASGA) and monopotassium salts (MPSGA) in various molar ratios were obtained. The physicochemical and spectral characteristics of the resulting complex compounds have been studied. Screening studies were carried out to study the specific hemostatic activity of the obtained supramolecular complexes. Among Lagochilin Lagochirzine and their derivatives at a dose of 10 mg/kg, Lagochilin had the most active effect on bleeding time, followed by Anhydrolagochilin–Lagohirzin. The greatest effect on bleeding time at a dose of 10 mg/kg among supramolecular complexes with HA was shown by HA-AHL, which in a ratio of 2 : 1 it was impossible to determine the bleeding time, and in a ratio of 4 : 1 it reduced the bleeding time by 12 times, and the amount of blood loss by 42 times.
Downloads
Metrics
References
Zaytsegub op'yanyayushchiy [Zaytsegub intoxicating]. URL: http://www.fito.nnov.ru/special/vitamines/lagochilus_inebrians/. (in Russ.).
Lagochilus inebrians Bunge // Plantarium. Rasteniya i lishayniki Rossii i sopredel'nykh stran: otkrytyy onlayn atlas i opredelitel' rasteniy. [Lagochilus inebrians Bunge // Plantarium. Plants and lichens of Russia and neighboring coun-tries: an open online atlas and guide to plants]. URL: https://www.plantarium.ru/page/view/item/21866.html. (in Russ.).
Zaynutdinov U.N., Islamov R., Dalimov D.N., Matchanov A.D., Abdurakhmanov T.R., Vypova N.L. Khimiya pri-rodnykh soyedineniy, 2002, no. 2, pp. 135–136. (in Russ.).
Matcanov A.D. Lagoxilinning fiziologik faol hosilalari sintezi: diss. kimjo fan. Nom. [Synthesis of physiologically ac-tive derivatives of lagoquiline: diss. chemistry Name]. Tashkent, 2003, pp. 25–40. (in Uzb.).
Dalimov D.N., Zaynutdinov U.N., Matchanov A.D., Musayev U.N., Mukhamadiyev M.G., Yuldashev Kh.A. Uzbekskiy khimicheskiy zhurnal, 2001, no. 5, pp. 33–36. (in Russ.).
Zaynutdinov U.N., Dalimov D.N., Yunusov T.K., Matchanov A.D. Khimiya prirodnykh soyedineniy, 2000, no. 3, pp. 225–226. (in Russ.).
Dalimov D.N., Matchanov A.D., Islamov A.H., Dalimov Sh.I., Sobirova F.A., Vipova N.L. Abstracts, 7 th Interna-tional Symposium on the Chemist ry of Natural Compounds. Urumqi Xinjiang China, 2011, p. 51.
Tolstikov G.A., Baltina L.A., Shul'ts E.E., Pokrovskiy A.G. Bioorganicheskaya khimiya, 1997, pp. 691–709. (in Russ.).
Turayeva D.T., Vypova N.L., Dalimova S.N., Dalimov D.N., Matchanov O.D., Niyazimbetova D. Vestnik NUUZ, 2005, pp. 93–95. (in Russ.).
Belozerskaya G.G., Makarov V.A., Aboyants R.K. i dr. Problemy gematologii, 2002, no. 2, pp. 17–22. (in Russ.).
Brekhov Ye.I., Severtsev A.N., Repin I.G. Farmakologicheskiye sredstva gemostaza v khirurgii pecheni: metodicheskiye rekomendatsii. [Pharmacological means of hemostasis in liver surgery: guidelines]. Moscow, 2005, 33 p. (in Russ.).
Abzhuyeva O.V., Rusanov V.M., Zhidkov I.L. Gematologiya i transfuziologiya, 2000, vol. 45, no. 1, pp. 35–37. (in Russ.).
Matchanov O.D., Vypova N.L., Dalimov D.N., Turayev A.S., Gafurov M.B., Filatova A.V. Tez. konf. «Aktual'nyye problemy khimii pri rodnykh soyedineniy». [Tez. conf. "Actual Problems of the Chemistry of Natural Compounds"]. Tashkent, 2009, p. 192. (in Russ.).
Azimova N.A., Vypova N.L., Dalimov D.I. 6-y Slavyano-Baltiyskiy nauchnyy forum. [6th Slavic-Baltic Scientific Fo-rum]. St.-Petersburg, 2004, p. 8. (in Russ.).
Spravochnik Vidal'. Lekarstvennyye preparaty v Rossii. [Vidal's handbook. Medicines in Russia]. Moscow, 2005, 565 p. (in Russ.).
Vypova N.L., Niyazimbetova D.M., Dalimov D.N., Matchanov A.D., Gafurov M.B., Azimova N. Pharmaceutical Bulletin of Uzbekistan, 2006, no. 1, pр. 34−35.
Nikitin Yu.P., Tolstikov G.A., Ragino Yu.I., Lyakhovich V.V., Vavilin V.A., Makarova S.I., Safronova O.G., Sala-khutdinov N.F., Stakhneva Ye.M. Byulleten' eksperimental'noy biologii i meditsiny, 2008, no. 8, pp. 171–174. (in Russ.).
Torkhovskaya T.I., Ipatova O.M., Prozorovskiy V.N., Zakharova T.S., Zykova M.G Biomeditsinskaya khimiya, 2009, no. 2, pp. 185–193. (in Russ.).
Turayeva D.T., Vypova N.L., Dalimova S.N., Dalimov D.N., Matchanov A.D., Niyazimbetova D. Doklady AN RUz, 2008, no. 1, pp. 43–46. (in Russ.).
Turayeva D.T., Vypova N.L., Dalimova S.N. i dr. Vestnik NUUz, 2005, no. 4, pp. 99–101. (in Russ.).
Copyright (c) 2022 chemistry of plant raw material

This work is licensed under a Creative Commons Attribution 4.0 International License.

This work is licensed under a Creative Commons Attribution 4.0 International License.
The authors, which are published in this journal, agree to the following conditions:
1. Authors retain the copyright to the work and transfer to the journal the right of the first publication along with the work, at the same time licensing it under the terms of the Creative Commons Attribution License, which allows others to distribute this work with the obligatory indication of the authorship of this work and a link to the original publication in this journal .
2. The authors retain the right to enter into separate, additional contractual agreements for the non-exclusive distribution of the version of the work published by this journal (for example, to place it in the university depository or to publish it in a book), with reference to the original publication in this journal.
3. Authors are allowed to post their work on the Internet (for example, in a university repository or on their personal website) before and during the review process of this journal, as this may lead to a productive discussion, as well as more links to this published work.











