STUDY OF ISOTHERMS OF WATER VAPOR SORPTION FOR HYDROPHILIC POLYMERS
UDC 547.458+678.744:544.723
Аннотация
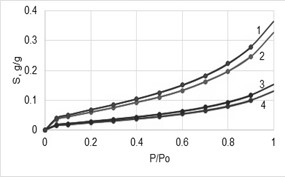
Various models and equations of water vapor (WV) sorption for hydrophilic polymers were considered. However, these models often do not correspond to the sorption mechanism. This study is based on the thermodynamics in binary systems and the Van Krevelen method of polar group contributions in the sorption of WV. Moreover, it was shown that the mechanism of WV sorption by various hydrophilic polymers is the absorption of water molecules in the volume of amorphous domains of these polymers. As a result, a universal physicochemical equation was proposed allowing adequately to describe the sorption isotherms of WV by amorphous hydrophilic polymers knowing only the chemical formulas of repeating units of these polymers. To calcu-late the sorption isotherms for semicrystalline polymer samples, it is necessary to use an additional parameter, namely the degree of amorphicity (Y). The adequacy of the derived equation was verified for samples of cellulose and other natural polysaccharides, as well as for samples of synthetic hydrophilic polymers such as polyvinyl alcohol, polyamide-6, and polycaprolactone having various Y-values. The verification showed that the experimental isotherms are almost identical to the isotherms calculated by the universal equation.
Скачивания
Metrics
Литература
Sionkowska A. Progress in Polym. Sci., 2011, vol. 36, pp. 1254–1276. DOI: 10.1016/j.progpolymsci.2011.05.003.
Sazali N., Ibrahim H., Jamaludin A.S. et al. Mater. Sci. Eng., 2020, vol. 788, pp. 1–15. DOI: 10.1088/1757-899X/788/1/012047.
Schmidt B. Polymers, 2019, vol. 11, pp. 693–698. DOI: 10.3390/polym11040693.
Grunin Y.B., Grunin L.Y., Schiraya V.Y. et al. Biores. Bioprocess, 2020, vol. 7, pp. 1–11. DOI: 10.1186/s40643-020-00332-8.
Andrade R.D., Lemus R., Pérez C. Vitae, 2011, vol. 18, pp. 325–334.
Papkov S.P., Fainberg E.Z. Vzaimodeystviye tsellyulozy i tsellyuloznykh materialov s vodoy. [Interaction of cellulose and cellulosic materials with water]. Moscow, 1976, 232 p. (in Russ.).
Timmermann E.O. Colloid Surface, 2003, vol. 220, pp. 235–260. DOI: 10.1016/S0927-7757(03)00059-1.
Czepirsky L., Komarowska-Czepirska E., Szymonska J. Appl. Surface. Sci., 2002, vol. 196, pp. 150–153. DOI: 10.1016/S0169-4332(02)00050-8.
Brousse M.M., Linares R.A., Vergara M.L., Nieto A.B. RECyT, 2017, vol. 19, pp. 29–37.
Blahovec J., Yanniotis S. Food Bioprocess Technol., 2008, vol. 1, pp. 82–90. DOI: 10.1007/s11947-007-0012-3.
Roja J., Moren S., Lopez A. J. Pharm. Sci. Res., 2011, vol. 3, pp. 1302–1309.
Broudin M., Le Saux V., Le Gac Pierre-Yves L. et al. Polymer Testing, 2015, vol. 171, pp. 87–95. DOI: 10.1016/j.polymertesting.2015.02.004.
Hill C.A.S., Norton A., Newman G. J. Appl. Polym. Sci., 2009, vol. 112, pp. 1524–1537. DOI: 10.1002/app.29725.
Park G.S. Synthetic membranes: science, engineering and applications. Springer: Dordrecht, 1986, vol 181, pp. 57–107.
Bessadok A., Langevin D., Gouanvé F. et al. Carbohyd. Polym., 2009, vol. 76, pp. 74–85. DOI: 10.1016/j.carbpol.2008.09.033.
Chalykh A.E., Bardyshev I.I., Petrova T.F. Polymers, 2021, vol. 13, pp. 2644–2658. DOI: 10.3390/polym13162644.
Ostrovskii V.E., Tsurkova B.V. Thernrochim. Acta, 1998, vol. 316, pp. 111–122.
Caulfield D.F., Steffes R.A. TAPPI, 1989, vol. 52, pp. 1361–1367.
Paes S.S., Sun Sh., MacNaughtan W. et al. Cellulose, 2010, vol. 17, pp. 693–709. DOI: 10.1007/s10570-010-9425-7.
Parodi E. Structure properties relations for polyamide-6. Univ Press: Eindhoven, 2017, 118 p.
Prusov A.N., Prusova S.M., Radugin M.V., Zakharov A.G. Russ. J. Phys. Chem., 2014, vol. 88, pp. 813–818.
Ioelovich M. SITA, 2016, vol. 18, pp. 35–42.
Ioelovich M., Leykin A. Bioresources, 2011, vol. 6, pp. 178–195. DOI: 10.15376/biores.6.1.178-195.
Van Krevelen D.W., Nijenhuis K. Properties of Polymers: Correlations with chemical structure. Elsevier: Amsterdam, 2009, 1004 p.
Ioelovich M. Organic Polym. Mater. Res., 2021, vol. 3, pp. 12–23. DOI: 10.30564/opmr.v3i2.4181.
Copyright (c) 2022 Химия растительного сырья

Это произведение доступно по лицензии Creative Commons «Attribution» («Атрибуция») 4.0 Всемирная.

This work is licensed under a Creative Commons Attribution 4.0 International License.
Авторы, которые публикуются в данном журнале, соглашаются со следующими условиями:
1. Авторы сохраняют за собой авторские права на работу и передают журналу право первой публикации вместе с работой, одновременно лицензируя ее на условиях Creative Commons Attribution License, которая позволяет другим распространять данную работу с обязательным указанием авторства данной работы и ссылкой на оригинальную публикацию в этом журнале.
2. Авторы сохраняют право заключать отдельные, дополнительные контрактные соглашения на неэксклюзивное распространение версии работы, опубликованной этим журналом (например, разместить ее в университетском хранилище или опубликовать ее в книге), со ссылкой на оригинальную публикацию в этом журнале.
3. Авторам разрешается размещать их работу в сети Интернет (например, в университетском хранилище или на их персональном веб-сайте) до и во время процесса рассмотрения ее данным журналом, так как это может привести к продуктивному обсуждению, а также к большему количеству ссылок на данную опубликованную работу.











