THE STUDY OF ANTIMICROBIAL AND ANALGESIC ACTIVITY OF CERTAIN CYTIZINE ALKALOID COMBINED DERIVATIVES
UDC 547.973: 615.1
Abstract
The modern vegetable matter studies in the republic and abroad are devoted to a multi-faceted and comprehensive research of plant raw materials, including desorbing and structure of plant component chemical properties, the study of their biological activity, as well as the development of effective and environmentally friendly methods for соmprehensive mineral processing of plant raw materials.
The 1,2,3-triazoles attraction is due to their reactivity versatility, as well as practical application of 1,2,3-triazole derivatives as medicines. Modification of natural compound molecules by introducing such a substituent is one of promising directions in the search for new biologically active compounds.
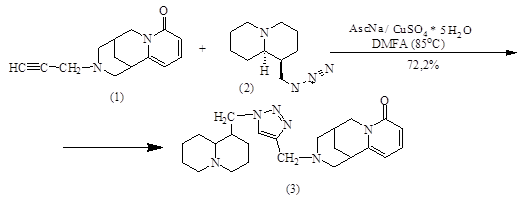
According to the data obtained, the antimicrobial activity of sample (5) is higher than that of sample (3). Thus, sample (5) showed a moderate antibacterial activity against Escherichia coli, whereas sample (3) showed a weak antimicrobial activity against this test strain.
The analysis of test results concerning the analgesic activity assessment showed that samples (5) and (3) can reduce the rats ‘specific nociceptive responses severity when testing abdominal constriction. It is important to emphasize that when injecting a 1% acetic acid solution abdominally, all test animals developed “acetic writhing’s” (characteristic animal movements, including the abdominal muscles contraction, alternating with their relaxation, hind limbs stretching and back arching).
Downloads
Metrics
References
Prakash B. Kujur A., Yadav A. Synthesis of Medicinal Agents from Plant. Elsevier Ltd., 2018, pp. 25–46. DOI: 10.1016/b978-0-08-102071-5.00002-7.
Thomford N.E., Senthebane D.A., Rowe A., Munro D., Seele P., Maroyi A., Dzobo K. Int. J. Mol. Sci., 2018, vol. 19, no. 6, p. 1578. DOI: 10.3390/ijms19061578.
Heinrich M., Mah J., Amirkia V. Molecules, 2021, vol. 26, no. 7, p. 1836. DOI: 10.3390/molecules26071836.
Ng Y.P., Or T.C.T., Ip N.Y. Neurochemistry International, 2015, vol. 89, pp. 260–270. DOI: 10.1016/j.neuint.2015.07.018.
Bondarenko S.P., Frasinyuk M.S., Vinogradova V.I., Khilya V.P. Khimiya prirodnykh soyedineniy, 2011, no. 4, pp. 536–538. (in Russ.).
Bondarenko S.P., Frasinyuk M.S., Vinogradova V.I., Khilya V.P. Khimiya prirodnykh soyedineniy, 2010, no. 5, pp. 649–651. (in Russ.).
Frasinyuk M.S., Turov A.V., Vinogradova V.I., Khilya V.P. Khimiya prirodnykh soyedineniy, 2007, no. 3, pp. 237–241. (in Russ.).
Frasinyuk M.S., Turov A.V., Vinogradova V.I., Khilya V.P. Khimiya prirodnykh soyedineniy. 2007, no. 2, pp. 145–148. (in Russ.).
Tlegenov R.T., Aytmagambetov A. Bioorganicheskaya khimiya, 2005, pp. 549–552. (in Russ.).
Yadav P.P., Gupta P., Chaturvedi A.K., Shukla P.K., Maurya R. Bioorganic & Medicinal Chemistry, 2005, vol. 13, no. 5, pp. 1497–1505.
Rouden J., Lasne M.-C., Blanchet J., Baudoux J. Chemical Reviews, 2013, vol. 114, no. 1, pp. 712–778. DOI: 10.1021/cr400307e.
Makara N.S., Gabdrakhmanova S.F., Sapozhnikova T.A., Khisamutdinova R.Y., Koval’skaya A.V., Tsypysheva I.P., Zarudii F.S. Pharmaceutical Chemistry Journal, 2015, vol. 49, no. 5, pp. 301–303. DOI: 10.1007/s11094-015-1283-z.
Tasso B., CanuBoido C., Terranova E., Gotti C., Riganti L., Clementi F., Artali R., Bombieri G., Meneghetti F., Spara-tore F. Journal of Medicinal Chemistry, 2009, vol. 52, no. 14, pp. 4345–4357. DOI: 10.1021/jm900225j.
Marrière E., Rouden J., Tadino V., Lasne M.-C. Organic Letters, 2000, vol. 2, no. 8, pp. 1121–1124. DOI: 10.1021/ol005685m.
Taly A., Corringer P.-J., Guedin D., Lestage P., Changeux, J.-P. Nature Reviews Drug Discovery, 2009, vol. 8, no. 9, pp. 733–750. DOI: 10.1038/nrd2927.
Molyneux R.J., Panter K.E. The Alkaloids: Chemistry and Biology. Amsterdam, Boston, Heidelberg, London, New York, Oxford, Paris, San Diego, San Francisco, Sydney, Tokyo, Academic Press, 2009, pp. 143–216. DOI: 10.1016/s1099-4831(09)06703-0.
Kamarul Zaman M.A., Azzeme A.M. GSC Biological and Pharmaceutical Sciences, 2019, vol. 06, no. 2, pp. 21–29. DOI: 10.30574/gscbps.2019.6.2.0003.
Golobokova T.V., Proydakov A.G., Kizhnyayev V.N. Zhurnnal organicheskoy khimii, 2020, pp. 442–450. DOI: 10.31857/S0514749220030143. (in Russ.).
Nurkenov O.A., Baykenova G.G., Turdybekov D.M., Ibrayev M.K., Gazaliyev A.M., Turdybekov K.M. Zhurnal ob-shchey khimii, 2006, vol. 76, no. 1, pp. 132–134. (in Russ.).
Brel V.K. Russian Journal Organic Chemistry, 2016, vol. 52, no. 1, pp. 54–60.
Artyushin O.I., Moiseeva A.A., Zarubaev V.V., Slita A.V., Galochkina A.V., Muryleva A.A., Borisevich S.S., Ya-rovaya O.I. Chemistry Biodiversity, 2019, vol. 16, no. 11, e1900340. DOI: 10.1002/cbdv.201900340.
Khabriyev R.U. Rukovodstvo po eksperimental'nomu (doklinicheskomu) izucheniyu novykh farmakologicheskikh vesh-chestv. [Guidelines for the experimental (preclinical) study of new pharmacological substances]. Moscow, 2005, 832 p. (in Russ.).
Gosudarstvennaya farmakopeya Respubliki Kazakhstan. [State Pharmacopoeia of the Republic of Kazakhstan]. Al-maty, 2008, vol. 1, 592 p. (in Russ.).
Copyright (c) 2022 chemistry of plant raw material

This work is licensed under a Creative Commons Attribution 4.0 International License.

This work is licensed under a Creative Commons Attribution 4.0 International License.
The authors, which are published in this journal, agree to the following conditions:
1. Authors retain the copyright to the work and transfer to the journal the right of the first publication along with the work, at the same time licensing it under the terms of the Creative Commons Attribution License, which allows others to distribute this work with the obligatory indication of the authorship of this work and a link to the original publication in this journal .
2. The authors retain the right to enter into separate, additional contractual agreements for the non-exclusive distribution of the version of the work published by this journal (for example, to place it in the university depository or to publish it in a book), with reference to the original publication in this journal.
3. Authors are allowed to post their work on the Internet (for example, in a university repository or on their personal website) before and during the review process of this journal, as this may lead to a productive discussion, as well as more links to this published work.











