ANTHOCYANINS IN LILAC FLOWERS SYRINGA VULGARIS
UDC 543.544.123:543.51:577.13
Abstract
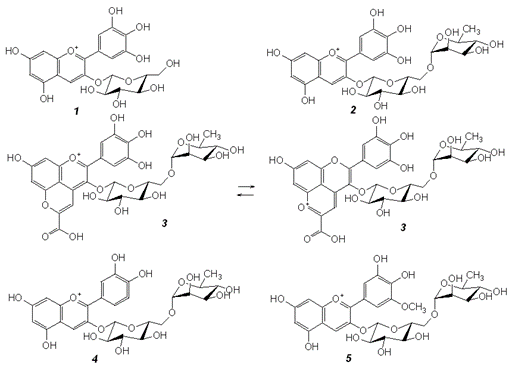
Anthocyanins from flowers of common lilac (Syringa vulgaris L.) of various color intensities and shades of lilac color from nine samples purchased at the Belgorod market were studied using reverse-phase high-performance liquid chromatography. To determine the structure of anthocyanins, we used the analysis of electronic absorption spectra recorded in the cuvette of a diode array detector and the analysis of mass spectra obtained by electrospray ionization with partial fragmentation. As a result, it was found that in all the studied samples the main component was delphinidin-3-rutinoside (84–90% by peak areas in the chromatogram). The level of cyanidin-3-rutinoside biosynthesis was significantly lower (6–19.6%). Among the minor compounds, delphinidin-3-glucoside and petunidin-3-glucoside were found. Among the unusual compounds, pyranoanthocyanin, built on the basis of delphinidin-3-rutinoside due to condensation with pyruvic acid, was found in a number of studied samples, but the reasons for its appearance have not yet been established. The total content of anthocyanins is low and amounts to 0.020–0.120 g per 100 g of fresh material (depending on the color intensity of the original plant material) in terms of cyanidin-3-glucoside. By drying flowers on a cut branch, air-dried material was obtained containing 0.100–0.300 g per 100 g of anthocyanins.
Downloads
Metrics
References
Tóth G., Barabás C., Tóth A., Kéry Á., Béni S., Boldizsár I., Varga E., Noszál B. Biomed. Chromatogr., 2016, vol. 30, pp. 923–932. DOI: 10.1002/bmc.3630.
Deeva A.M., Shabunya P.S., Fatykhova S.A., Zubarev A.V., Reshetnikov V.N., Spiridovich E.V. Fiziologiya rasteniy, 2022, vol. 69, no. 2, pp. 161–170. DOI: 10.31857/S001533032202004X. (in Russ.).
Deineka V.I., Sidorov A.N., Deineka L.A., Kostenko M.O., Blinova I.P. Russ. J. Phys. Chem. A, 2016, vol. 90, no. 4, pp. 861–863. DOI: 10.1134/S0036024416040075.
Welch C.R., Wu Q., Simon J.E. Curr. Anal. Chem., 2008, vol. 4, pp. 75–101. DOI: 10.2174/157341108784587795.
Khoo H.E., Azlan A., Tang S.T., Lim S.M. Food Nutr. Res., 2017, vol. 61, article 1361779. DOI: 10.1080/16546628.2017.1361779.
Fossen T., Cabrita L., Andersen Ø.M. Food Chem., 1998, vol. 63, no. 4, pp. 435–440. DOI: 10.1016/S0308-8146(98)00065-X.
Castañeda-Ovando A., Pacheco-Hernández M., Páez-Hernández M.E., Rodríguez J.A., Galán-Vidal C.A. Food Chem., 2009, vol. 113, pp. 859–871. DOI: 10.1016/j.foodchem.2008.09.001
Deng R.-X., Yuan H., Liu P., Yin W.-P., Wang X.-S., Zhao T.-Z. Biochem. System. Ecol., 2010, vol. 38, pp. 813–815. DOI: 10.1016/j.bse.2010.08.004.
Su G., Cao Y., Li C., Yu X., Gao X., Tu P., Chai X. Chem. Central J., 2015, vol. 9, article 2. DOI: 10.1186/s13065-015-0079-2.
Woźniak M., Michalak B., Wyszomierska J., Dudek M.K., Kiss A.K. Front Pharmacol., 2018, vol. 9, article 349. DOI: 10.3389/fphar.2018.00349.
Mlcek J., Rop O. Trends Food Sci. Technol., 2011, vol. 22, pp. 561–569. DOI: 10.1016/j.tifs.2011.04.006
Deineka V.I., Sidorov A.N., Chulkov A.N., Deineka L.A. J. Anal. Chem., 2017, vol. 72, no. 14, pp. 1441–1445. DOI: 10.1134/S1061934817140040.
Giusti M.M., Wrolstad R.E. Curr. Prot. Food Anal. Chem., 2001, pp. F1.2.1–F1.2.13. DOI: 10.1002/0471142913.faf0102s00.
Deyneka L.A., Shaposhnik Ye.I., Gostishchev D.A., Deyneka V.I., Sorokopudov V.N., Selemenev V.F. Sorbtsionnyye i khromatograficheskiye protsessy, 2009, vol. 9, no. 4, pp. 529–536. (in Russ.).
Zhao Xu. He X.-M., Liud F.., Duan C.-Q., He F. Food Chem., 2022, vol. 391, article 133255. DOI: 10.1016/j.foodchem.2022.133255.
Harborne J.B. Biochem. J., 1958, vol. 70, pp. 22–28. DOI: 10.1042/bj0700022.
Deyneka V.I., Salasina Ya.Yu., Blinova I.P., Deyneka L.A., Varushkina S.M., Chulkov A.N., Selemenev V.F. Sor-btsionnyye i khromatograficheskiye protsessy, 2021, vol. 21, vol. 2, pp. 187–195. DOI: 10.17308/sorpchrom.2021.21/3353. (in Russ.).
He F., Liang N.-N., Mu L., Pan Q.-H., Wang J., Reeves M.J., Duan C.-Q. Molecules, 2012, vol. 17, pp. 1483–1519. DOI: 10.3390/molecules17021483.
Copyright (c) 2023 chemistry of plant raw material

This work is licensed under a Creative Commons Attribution 4.0 International License.

This work is licensed under a Creative Commons Attribution 4.0 International License.
The authors, which are published in this journal, agree to the following conditions:
1. Authors retain the copyright to the work and transfer to the journal the right of the first publication along with the work, at the same time licensing it under the terms of the Creative Commons Attribution License, which allows others to distribute this work with the obligatory indication of the authorship of this work and a link to the original publication in this journal .
2. The authors retain the right to enter into separate, additional contractual agreements for the non-exclusive distribution of the version of the work published by this journal (for example, to place it in the university depository or to publish it in a book), with reference to the original publication in this journal.
3. Authors are allowed to post their work on the Internet (for example, in a university repository or on their personal website) before and during the review process of this journal, as this may lead to a productive discussion, as well as more links to this published work.











