INDIRECT ELECTROCATALYTIC OXIDATION OF STARCH BY REACTIVE OXYGEN SPECIES IN SITU GENER-ATED ON Pb/PbO2 ANODE AND BORON-DOPED DIAMOND ELECTRODE IN AQUEOUS ELECTROLYTES
UDC 541.135:547.0
Abstract
The indirect electrocatalytic oxidation of starch by reactive oxygen species (ROS) generated in situ on lead dioxide anodes and a boron-doped diamond electrode in an acidic aqueous electrolyte has been studied. The influence of the current density, concentration and state of aggregation of the reagent, the scheme of introduction of ROS on the kinetics and yields of the product of indirect oxidation of starch dialdehyde karazmal has been established. The optimal conditions for obtaining oxidized starch were determined: anode – Pb/PbO2, current density – 25 mA/cm-2, electrolyte pH 2–3, electrolysis time – 80 min, 25 °C. Starch oxidation products were analyzed by the following methods: spectrophotometry after deratization with dinitrophenylidrazine, gas chromatography-mass spectrometry after hydrolysis and silylation, and IR spectroscopy. Glucose tautomers, as well as ethylene glycol oligomers, were found in the hydrolyzates of the reaction products by GCMS, indicating the breaking of C-C bonds in the monosaccharide units during the oxidation process.
Downloads
Metrics
References
Valeyeva N.Sh., Khasanova G.B. Vestnik Kazanskogo tekhnologicheskogo universiteta, 2013, vol. 16, no. 22, pp. 184–187. (in Russ.).
Rus'kina A.A., Popova N.V., Naumenko N.V., Rus'kin D.V. Vestnik Yuzhno-Ural'skogo gosudarstvennogo universi-teta. Seriya: Pishchevyye i biotekhnologii, 2017, vol. 5, no. 3, pp. 12–20. DOI: 10.14529/food170302. (in Russ.).
Beletskaya I.P., Kustov L.M. Uspekhi khimii, 2010, vol. 79, no. 6, pp. 493–515. (in Russ.).
Korniyenko V.L., Kolyagin G.A., Korniyenko G.V., Kenova T.A. Elektrokhimiya, 2020, vol. 56, no. 5, pp. 427–441. DOI: 10.31857/S0424857020050060. (in Russ.).
Zarei M., Niaei А., Salari D. Journal of electroanalytical chemistry, 2010, vol. 639, no. 1-2, pp. 167–174. DOI: 10.1016/j.jelechem.2009.12.005.
Korniyenko V.L. Khimiya v interesakh ustoychivogo razvitiya, 2002, vol. 10, p. 391. (in Russ.).
Ogibin Yu.N., Elinson M.N., Nikishin G.I. Uspekhi khimii, 2009, vol. 78, p. 99. (in Russ.).
Brillas E. et al. Journal of the Electrochemical Society, 1995, vol. 142, no. 6, p. 1733. DOI: 10.1149/1.2044186.
Nidheesh P.V., Zhou M., Oturan M.A. Chemosphere, 2018, vol. 197, pp. 210–227. DOI: 10.1016/j.chemosphere.2017.12.195.
Dang X. et al. International journal of biological macromolecules, 2019, vol. 121, pp. 113–119. DOI: 10.1016/j.ijbiomac.2018.10.012.
Li D. et al. Journal of Industrial Engineering Chemistry, 2022, vol. 105, pp. 405–413.
Comninellis C. Electrochimica Acta, 1994, vol. 39, no. 11-12, pp. 1857–1862. DOI: 10.1016/0013-4686(94)85175-1.
Martinez-Huitle C.A., Ferro S. Chemical Society Reviews, 2006, vol. 35, no. 12, pp. 1324–1340. DOI: 10.1039/B517632H.
Li D. et al. Journal of hazardous materials, 2020, vol. 396, 122591.
Dang X. et al. International journal of biological macromolecules, 2019, vol. 121, pp. 113–119. DOI: 10.1016/j.ijbiomac.2018.10.012.
Butrim S.M., Bil'dyukevich T.D., Butrim N.S., Yurkshtovich T.L. Izvestiya vysshikh uchebnykh zavedeniy. Khimiya i khimicheskaya tekhnologiya, 2015, vol. 58, no. 1, pp. 28–32. (in Russ.).
Parovuori P. Hamunen A., Forssell P., Autio K. Starch-Stärke, 1995, vol. 47, no. 1, pp. 19–23. DOI: 10.1002/star.19950470106.
Tolvanen P., Mäki-Arvela P., Sorokin A.B., Salmi T., Murzin D.Yu. Chemical Engineering Journal, 2009, vol. 154, no. 1-3, pp. 52–59. DOI: 10.1016/j.cej.2009.02.001.
Fioshin M.Ya., Smirnova M.G. Elektrokhimicheskiye sistemy v sinteze khimicheskikh produktov. [Electrochemical sys-tems in the synthesis of chemical products]. Moscow, 1985, 256 p. (in Russ.).
Korniyenko G.V., Kapayeva S.N., Malyar Yu.N., Korniyenko V.L., Taran O.P. Khimiya rastitel'nogo syr'ya, 2021, no. 4, pp. 119–127. DOI: 10.14258/jcprm.20210410590. (in Russ.).
Shamb U., Setterfild Ch., Ventvors R. Perekis' vodoroda. [Hydrogen peroxide]. Moscow, 1958, 578 p. (in Russ.).
Ruiz-Matute A., Hernandez-Hernandez O., Rodríguez-Sánchez S., Sanz M., Martínez-Castro I. Journal of chromatog-raphy B, 2010, vol. 879, pp. 1226–1240. DOI: 10.1016/j.jchromb.2010.11.013.
Abd El Aal E.E. Journal of power sources, 2001, vol. 102, no. 1-2, pp. 233–241. DOI: 10.1016/S0378-7753(01)00804-7.
Zhang Y.R., Zhang S.D., Wang X.L., Chen R.Y., Wang Y.Z. Carbohydrate Polymers, 2009, vol. 78, no. 1, pp. 157–161. DOI: 10.1016/j.carbpol.2009.04.023.
Brillas E., Martínez-Huitle C.A. Electrochemistry, Characterization, and Applications, 2011, vol. 8.
Damodkhar G., Prabir G. Elektrokhimiya, 2019, vol. 55, no. 7, pp. 771–807. DOI: 10.1134/S0424857019050050. (in Russ.).
Ribeiro L.S., Órfão J.J.M., Pereira M.F.R. Bioresource Technology, 2018, vol. 263, pp. 402–409.
Gromov N.V., Zhdanok A.A., Medvedeva T.B., Lukoyanov I.A., Poluboyarov V.A., Taran O.P., Parmon V.N., Timofeeva M.N. Catalysis in Industry, 2020, vol. 12, no. 4, pp. 343–352.
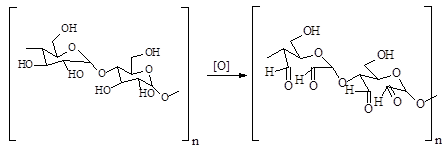
Copyright (c) 2022 chemistry of plant raw material

This work is licensed under a Creative Commons Attribution 4.0 International License.

This work is licensed under a Creative Commons Attribution 4.0 International License.
The authors, which are published in this journal, agree to the following conditions:
1. Authors retain the copyright to the work and transfer to the journal the right of the first publication along with the work, at the same time licensing it under the terms of the Creative Commons Attribution License, which allows others to distribute this work with the obligatory indication of the authorship of this work and a link to the original publication in this journal .
2. The authors retain the right to enter into separate, additional contractual agreements for the non-exclusive distribution of the version of the work published by this journal (for example, to place it in the university depository or to publish it in a book), with reference to the original publication in this journal.
3. Authors are allowed to post their work on the Internet (for example, in a university repository or on their personal website) before and during the review process of this journal, as this may lead to a productive discussion, as well as more links to this published work.











