PECULIARITY OF PHASE TRANSITION CI INTO CII FOR NANOCRYSTALLITES OF CELLULOSE
Abstract
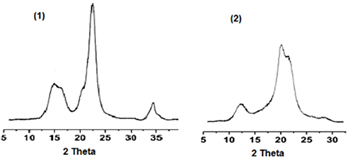
In this paper using a method of wide angle X-ray diffraction, sizes of cellulose nanoscale crystallites were determined and phase transition of nano-sized crystallites CI into CII was studied after treatment of cellulose samples with solutions of sodium hydroxide with various concentrations, 5 to 20% (1,3 to 6,1 M). It was found, the phase transition proceeds in a certain interval of hydroxide concentrations; besides, a correlation between average concentration (С) of hydroxide and average lateral sizes (D) of nanocrystallites was observed. Methods of chemical thermodynamics of nano-phases allowed to derive an equation, which describes the relationship between C and D: lnC = lnCo - KD-1, where Co is maximum concentration of hydroxide, which is required for the phase transition of large crystals of CI. Thus, the decrease in hydroxide concentration at the phase transition CI into CII, is explained by decreasing of lateral size of CI nanocrystallites. By means of the derived equation, minimum, average and maximum lateral sizes of CI nanocrystallites were determined, as well as polydispersity in lateral sizes of crystallites was studied. It has been shown that crystallites of organo-solvent celluloses were the most uniform, whereas aggregated crystallites of Kraft celluloses were the most heterogeneous.Downloads
Metrics
References
Serkov A.T. Viskoznye volokna. [Viscose fibers]. Moscow, 1981, 296 p. (in Russ.).
Ioelovich M.Ia., Karlivan V.P. Khimiia drevesiny, 1986, no. 1, pp. 18–25. (in Russ.).
Ioelovich M.Ia. Khimiia drevesiny, 1990, no. 2, pp. 8–15. (in Russ.).
Goikhman A.Sh., Solomko V.P. Vysokomolekuliarnye soedineniia vkliucheniia. [Macromolecular compounds inclusion]. Kiev, 1982, 192 p. (in Russ.).
Traiber E. Khimiia i tekhnologiia polimerov, 1967, no. 6, pp. 81–95. (in Russ.).
Ioelovich M.Ia., Veveris G.P. Khimiia drevesiny, 1984, no. 6, pp. 36–41. (in Russ.).
Rudholm S.A. Pulping processes, New York, London: Interscience, 1985, 1270 p.
Nishimura H., Sarko A. J. Appl. Polym. Sci., 1987, vol. 33, no. 3, pp. 867–874.
Ioelovich M.Ia., Veveris G.P. Khimiia drevesiny, 1986, no. 3, pp. 7–11. (in Russ.).
Ioelovich M., Leykin A., Figovsky O. Bioresources, 2010, vol. 5, no. 3, pp. 1393–1407.
Ioelovich M. Cellulose nanostructured natural polymer, Saarbrücken: LAP, 2014, 90 p.
Ioelovich M.Ia., Veveris G.P. Khimiia drevesiny, 1983, no. 2, pp. 10–14. (in Russ.).
Poltorak O.M. Termodinamika v fizicheskoi khimii. [Thermodynamics in Physical Chemistry]. Moscow, 1991, 320 p. (in Russ.).
Frolov Iu.G. Kurs kolloidnoi khimii: poverkhnostnye iavleniia i dispersnye sistemy. [Course of Colloid Chemistry: surface phenomena and disperse systems]. Moscow, 1982, 400 p. (in Russ.).
Perez M. Scripta Materialia, 2005, vol. 52, pp. 709–712.
Ioelovich M.Ia. Khimiia drevesiny, 1991, no. 4, pp. 27–33. (in Russ.).

This work is licensed under a Creative Commons Attribution 4.0 International License.
The authors, which are published in this journal, agree to the following conditions:
1. Authors retain the copyright to the work and transfer to the journal the right of the first publication along with the work, at the same time licensing it under the terms of the Creative Commons Attribution License, which allows others to distribute this work with the obligatory indication of the authorship of this work and a link to the original publication in this journal .
2. The authors retain the right to enter into separate, additional contractual agreements for the non-exclusive distribution of the version of the work published by this journal (for example, to place it in the university depository or to publish it in a book), with reference to the original publication in this journal.
3. Authors are allowed to post their work on the Internet (for example, in a university repository or on their personal website) before and during the review process of this journal, as this may lead to a productive discussion, as well as more links to this published work.











