FLAVONOIDS OF AERIAL PARTS OF SPREADING MARIGOLD (TAGETES PATULA L.)
UDC 615.322: 547.972 + 543.544
Abstract
The arial parts of the spreading marigold (Tagetes patula L.) of the Asteraceae family is a promising source of biologically active compounds, including flavonoids. It is known, that the content of total flavonoids in the flowers of this plant reaches 9% (calculated on patulitrin), and in the aboveground part (leaves and stems) – about 3.0% (calculated on rutin), however, data on the component composition of flavonoids are contradictory.
The aim of the study is to study the flavonoid component composition of the arial p part of the rejected marigolds.
As a result of studies using column chromatography on silica gel L 40/100, 6-methoxykaempferol (2), 7-O-glucoside of methoxykaempferol (3), quercetin (6), patuletin (7) and patulitrin (7-O-β-D-glucopyranoside of patuletin) (12) were isolated for the first time from the flowers of Tagetes patula L. (Mandarin variety), and from the arial parts (leaves and stems) of this species - kaempferitrin (3-O-α-L-rhamnopyranoside-7-O-α-L-rhamnopyranoside of kaempferol) (4), 3-O-β-D-xylopyranoside-7-O-α-L-rhamnopyranoside of kaempferol (5), quercetin (6), patuletin (7), 3-O-β-D-xylopyranoside-7-O-α-L-rhamnopyranoside of quercetin (8), 7-O-α-L-rhamnopyranoside of quercetin (9), quercitrin (10), isoquercitrin (11). Interestingly, that the common components of the herbs (leaves and stems) and flowers are only two flavonoids – quercetin (6) and patuletin (7). As for patulitrin (7-O-β-D-glucopyranoside of 3,5,7,3’,4’-pentahydroxy-6-methoxyflavone, or patuletin) (12), which is the dominant flavonoid of the flowers of this plant, this component is not found in the herbs of this plant. It was determined, that glycosides of kaempferol (1) and quercetin (6) predominate in the herb of the Tagetes patula L. with the dominant flavonoid being 3,7-O-dirhamnoside of kaempferol (4).
The identification of the isolated flavonoids was carried out using UV, 1H-NMR, 13C-NMR spectroscopy and mass spectrometry, as well as the results of acid and enzymatic hydrolysis.
3-O-β-D-xylopyranoside-7-O-α-L-rhamnopyranoside of kaempferol (5) and 3-O-β-D-xylopyranoside-7-O-α-L-rhamnopyranoside of quercetin (8), isolated from the herb of the Tagetes patula L., are new natural compounds.
Downloads
Metrics
References
Malyugina Ye.A., Mazulin A.V., Smoylovskaya G.P. Nauchnyye trudy SWorld, 2015, vol. 18, no. 2 (39), pp. 48–52. (in Russ.).
Podgornaya Zh.V. Issledovaniye tsvetkov barkhattsev rasprostertykh (Tagetes patula L.) s tsel'yu polucheniya bio-logicheski aktivnykh soyedineniy: dis. … kand. farm. nauk. [Study of prostrate marigold flowers (Tagetes patula L.) in order to obtain biologically active compounds: dis. ...cand. pharm. Sci.]. Pyatigorsk, 2008. (in Russ.).
Samosudova I.Ye., Boyko N.N., Tsvetkov Z.Ye. Sovremennyye tendentsii razvitiya tekhnologiy zdorov'yesberezheni-ya. [Modern trends in the development of health-saving technologies]. Moscow, 2019, pp. 315–319. (in Russ.).
Chervonnaya N.M. Traditsionnaya i innovatsionnaya nauka: istoriya, sovremennoye sostoyaniye, perspektivy. [Tradi-tional and innovative science: history, current state, prospects]. 2017, pp. 134–138. (in Russ.).
Chervonnaya N.M., Andreyeva O.A., Adzhiakhmetova S.L., Oganesyan E.T. Khimiya rastitel'nogo syr'ya, 2018, no. 3, pp. 91–98. (in Russ.).
Chervonnaya N.M., Andreyeva O.A., Kharchenko I.I. Nauchnyye vedomosti Belgorodskogo gosudarstvennogo universiteta. Seriya: Meditsina. Farmatsiya, 2016, no. 26 (247), pp. 147–151. (in Russ.).
Chervonnaya N.M., Oganesyan E.T., Andreyeva O.A., Senchenko S.P., Borovskiy B.V. Zdorov'ye i obrazovaniye v XXI veke, 2017, vol. 19, no. 6, pp. 132–137. (in Russ.).
Chervonnaya N.M., Kharchenko I.I., Adzhiakhmetova S.L., Mykots L.P., Andreyeva O.A., Oganesyan E.T. Farmatsiya i farmakologiya, 2017, vol. 5, no. 3, pp. 267–282. (in Russ.).
Benea A., Ciobanu C., Cojocaru-Toma M., Ciobanu N. The Moldovan Medical Journal, 2020, vol. 63, pp. 23–26.
Wang Yu.-M., Ran X.-K., Riaz M., Yu M., Cai Q., Dou D.-Q., Metwaly A.M., Kang T.-G., Cai D.-C. Molecules, 2019, vol. 24, p. 3911. DOI: 10.3390/molecules24213911.
Kurkina A.V., Savel'yeva A.Ye., Kurkin V.A. Khimiko-farmatsevticheskiy zhurnal, 2022, vol. 56, no. 5, pp. 43–46. (in Russ.).
Kurkina A.V., Savel'yeva A.Ye., Kurkin V.A. Khimiko-farmatsevticheskiy zhurnal, 2021, vol. 55, no. 2, pp. 46–50. (in Russ.).
Savel'yeva A.Ye., Kurkin V.A., Kurkina A.V. Farmatsiya, 2021, vol. 70, no. 6, pp. 24–30. (in Russ.).
Kurkin V.A., Savel'yeva A.Ye., Kurkina A.V. Khimiya rastitel'nogo syr'ya, 2022, no. 4, pp. 221–231. (in Russ.).
Deepshikha K., Yashodhara V. Annual Research & Review in Biology, 2017, vol. 13, pp. 1–8. DOI: 10.9734/ARRB/2017/34349.
Gongadze M., Machavariani M., Enukidze M., Gogia N., Iobadze M., Chkhikvishvili I. Georgian Med. News, 2019, vol. 297, pp. 154–157.
Meurer M.C., Mees M., Mariano L.N., Boeing T., Somensi L.B., Mariott M.M., Da Silva R.D., Dos Santos A.C.D., Longo B., França T.C.S., Klein-Junior L., de Souza P., de Andrade S.F., da Silva L.M. Nutrition research, 2019, vol. 66, pp. 95–106.
Lomkina Ye.M., Chervonnaya N.M., Kurkin D.V., Volotova Ye.V., Bakulin D.A., Oganesyan E.T., Andreyeva O.A., Tyurenkov I.N. Farmatsiya, 2016, vol. 65, no. 3, pp. 37–39. (in Russ.).
Papayani O.I., Dukhanina I.V., Sergeyeva O.Ye. Izvestiya Samarskogo nauchnogo tsentra Rossiyskoy akademii nauk, 2012, vol. 14, no. 5(3), pp. 742–744. (in Russ.).
Kholikova O., Azonov D.A., Ganiyev Kh.A. Colloquium-journal, 2019, no. 11-2 (35), pp. 49–52. (in Russ.).
Nawale S., Padma Priya K., Pranusha P., Ganga Raju M. Data in Brief, 2018, vol. 21, pp. 587–597.
Mabry T.J., Markham K.R., Thomas M.B. The Systematic Identification of Flavonoids. Berlin-Heidelberg-New York: Springer Verlag, 1970, 354 p.
Kurkina A.V. Flavonoidy farmakopeynykh rasteniy: monografiya. [Flavonoids of pharmacopoeial plants: monograph]. Samara, 2012, 290 p. (in Russ.).
Louaar S., Achouri A., Lefahal M., Laouer H., Medjroubi K., Duddeck H., Akkal S. Nat. Prod. Commun., 2011, vol. 7, pp. 1603–1604.
Louaar S., Zellagui A., Gherraf N., Medjroubi K., Derbre S., Seguin E., Laouer H., Akkal S. Dur. J. Biol. Act. Prod. Nat., 2014, vol. 4, pp. 249–253.
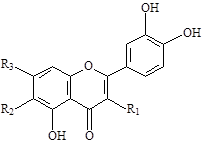
Copyright (c) 2023 chemistry of plant raw material

This work is licensed under a Creative Commons Attribution 4.0 International License.

This work is licensed under a Creative Commons Attribution 4.0 International License.
The authors, which are published in this journal, agree to the following conditions:
1. Authors retain the copyright to the work and transfer to the journal the right of the first publication along with the work, at the same time licensing it under the terms of the Creative Commons Attribution License, which allows others to distribute this work with the obligatory indication of the authorship of this work and a link to the original publication in this journal .
2. The authors retain the right to enter into separate, additional contractual agreements for the non-exclusive distribution of the version of the work published by this journal (for example, to place it in the university depository or to publish it in a book), with reference to the original publication in this journal.
3. Authors are allowed to post their work on the Internet (for example, in a university repository or on their personal website) before and during the review process of this journal, as this may lead to a productive discussion, as well as more links to this published work.











