OPTICALLY ACTIVE PINE SEX PHEROMONE SAWS: SYNTHESIS AND BIOLOGICAL ACTIVITY
UDK 632.79+547.268.1
Abstract
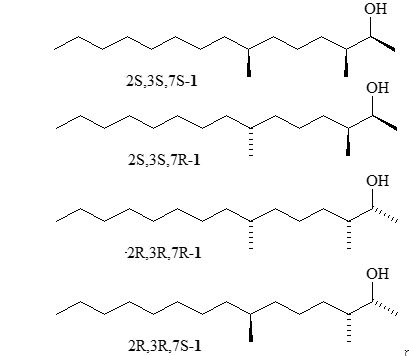
The most dangerous pest for coniferous trees, by right, can be called pine sawflies of the genera Diprion, Neodiprion and Gilpinia (Diprionidae) – insects that can cause tremendous harm to plants or even destroy them. Scots pines suffer the most from the pest, especially at the age of 20–40, however, ornamental plant species are often subject to mass attack by insects. The review article presents mechanical, biological and chemical methods of combating pine sawflies, the main part of the review is devoted to the use of the sex pheromone of males of these pests, namely, identification methods (isolation, physicochemical methods of analysis, electrophysiological experiments and field tests) of pine sawfly pheromone, stereoisomeric 3,7-dimethylpentadecan-1-ol (diprionol) acetate and propionate, and presents a list of publications on known syntheses of their racemic compounds forms, methods for controlling insect pests are described and all known chemical syntheses of possible stereomers of diprionol and its esters are presented individually or in a mixture with various degrees of optical purity. The review includes the following chapters: Introduction; The structure of the pine sawfly pheromone and its biological activity; Synthesis of stereoisomers of pine sawfly pheromone; Conclusion. The review includes 93 literature references.
Downloads
Metrics
References
Vorontsov A.I. Lesnaya entomologiya: Uchebnik dlya studentov lesokhoz. spets. vuzov. [Forest entomology: Textbook for students of specialized forestry universities]. Moscow, 1982, 384 p. (in Russ.).
Coppel H.C., Casida J.E., Dauterman W.C. Ann. Entomol. Soc. Am., 1960, vol. 53, no. 4, pp. 510–512. DOI: 10.1093/aesa/53.4.510.
Casida J.E., Coppel H. C., Watanabe T. J. Econ. Entomol., 1963, vol. 56, no. 1, pp. 18–24. DOI: 10.1093/jee/56.1.18.
Jewett D.M., Matsumura F., Coppel H.C. Science, 1976, vol. 192, no. 4234, pp. 51–53. DOI: 10.1126/science.1257754.
Jewett D.M., Matsumura F., Coppel H.C. J. Chem. Ecol., 1978, vol. 4, no. 3, pp. 277–287. DOI: 10.1007/BF00989337.
Matsumura F., Tai A., Coppel H.C., Imaida M. J. Chem. Ecol., 1979, vol. 5, no. 2, pp. 237–249. DOI: 10.1007/BF00988238.
Kocienski P.J., Ansell J.M. J. Org. Chem., 1977, vol. 42, no. 6, pp. 1102–1103, DOI: 10.1021/jo00426a045.
Mori K., Masuda S., Matsui M. Agric. Biol. Chem., 1978, vol. 42, no. 5, pp. 1015–1018. DOI: 10.1271/bbb1961.42.1015.
Place P., Roumestant M.-L., Gore J. J. Org. Chem., 1978, vol. 43, no. 5, p. 1001. DOI: 10.1021/jo00399a046.
Baker R., Winton P.M., Turner R.W. Tetrahedron Lett., 1980, vol. 21, no. 12, pp. 1175–1178. DOI: 10.1016/S0040-4039(01)83944-7.
Odinokov V.N., Akhmetova V.R., Ishmuratov G.Yu., Botsman L.P., Tolstikov G.A. Zhurnal organicheskoy khimii, 1986, vol. 22, no. 5, pp. 953–957. (in Russ.).
Odinokov V.N., Akhmetova V.R., Savchenko R.G. Khimiya prirodnykh soyedineniy, 1998, no. 1, pp. 123–126. (in Russ.).
Ishmuratov G.Yu., Kharisov R.Ya., Musavirov R.S., Zorin V.V., Rakhmankulov D.L., Odinokov V.N., Tolstikov G.A. Khimiya prirodnykh soyedineniy, 1997, no. 2, pp. 170–178. (in Russ.).
Odinokov V.N., Ishmuratov G.Yu., Kharisov R.Ya., Tolstikov G.A. Khimiya prirodnykh soyedineniy, 1989, no. 4, pp. 573–576. (in Russ.).
Odinokov V.N., Ishmuratov G.Yu., Ladenkova I.M., Tolstikov G.A. Khimiya prirodnykh soyedineniy, 1990, no. 6, pp. 818–822. (in Russ.).
Ishmuratov G.Yu., Yakovleva M.P., Galyautdinova A.V., Muslukhov R.R., Tolstikov G.A. Izv. AN. Ser. khim., 2003, no. 3, pp. 709–712. (in Russ.).
Odinokov V.N., Ishmuratov G.Yu., Ibragimov A.G., Yakovleva M.P., Zolotarev A.P., Dzhemilev U.M., Tolstikov G.A. Khimiya prirodnykh soyedineniy, 1992, no. 5, pp. 567–571. (in Russ.).
Serebryakov E.P., Gamalevich G.D. Izv. AN SSSR. Ser. khim., 1987, no. 1, pp. 114–118. (in Russ.).
Hedenström E., Högberg H.-E. Tetrahedron, 1994, vol. 50, no. 17, pp. 5225–5232. DOI: 10.1016/s0040-4020(01)90432-6.
Magnusson G. Tetrahedron, 1978, vol. 34, no. 9, pp. 1385–1388. DOI: 10.1016/0040-4020(78)88335-5.
Magnusson G. Tetrahedron Lett., 1977, vol. 18, no. 31, pp. 2713–2716. DOI: 10.1016/S0040-4039(01)83053-7.
Bestmann H.J., Vostrowsky O. Wittig Chemistry. Topics in Current Chemistry. Berlin, Heidelberg: Springer, 1983, vol. 109, pp. 85–163. DOI: 10.1007/BFb0018057.
Kallmerten J., Balestra M. J. Org. Chem., 1986, vol. 51, no. 14, pp. 2855–2857. DOI: 10.1021/jo00364a058.
Gould T.J., Balestra M., Wittman M.D., Gary J.A., Rossano L.T., Kallmerten J. J. Org. Chem., 1987, vol. 52, no. 17, pp. 3889–3901. DOI: 10.1021/jo00226a032.
Löfqvist J., Payne T.L., Birch M.C., Kennedy Eds C.E.J. Mechanisms in Insect Olfaction. Oxford: Oxford University Press, 1986. 364 p.
Kraemer M., Coppel H.C., Matsumura F., Kikukawa T., Mori K. Environ. Entomol., 1978, vol. 8, no. 3, pp. 519–520. DOI: 10.1093/ee/8.3.519.
Anderbrant O., Löfqvist J., Hedenström E., Bång J., Tai A., Högberg H.-E. J. Chem. Ecol., 2010, vol. 36, no. 9, pp. 969–977. DOI: 10.1007/s10886-010-9834-y.
Anderbrant O., Zhang Q.-H., Chu D. J. Appl. Entomol., 1997, vol. 121, no. 1-5, pp. 281–283. DOI: 10.1111/j.1439-0418.1997.tb01406.x.
Kraemer M.E., Coppel H.C., Matsumura F., Wilkinson R.C., Kikukawa T. J. Chem. Ecol., 1981, vol. 7, no. 6, pp. 1063–1072. DOI: 10.1007/BF00987628.
Kraemer M.E., Coppel H.C., Kikukawa T., Matsumura F., Thomas H.A., Thompson L.C., Mori K. Environ. Entomol., 1983, vol. 12, no. 5, pp. 1592–1596. DOI: 10.1093/ee/12.5.1592.
Kikukawa T., Matsumura F., Olaifa J., Kraemer M., Coppel H.C., Tai A. J. Chem. Ecol., 1983, vol. 9, no. 6, pp. 673–693. DOI: 10.1007/BF00988775.
Olaifa J.I., Matsumura F., Coppel H.C. J. Chem. Ecol., 1987, vol. 13, no. 6, pp. 1395–1408. DOI: 10.1007/BF01012286.
Anderbrant O., Löfqvist J., Högberg H.E., Hedenström E., Wassgren A.-B., Bergström G., Bengtsson M., Magnus-son G. Entomol. Exp. Appl., 1992, vol. 62, no. 2, pp. 169–181. DOI: 10.1111/j.1570-7458.1992.tb00657.x.
Vendilo N.V., Pletnev V.A., Lebedeva K.V., Maslov A.D., Komarova I.A., Seryy G.A. Lesnoy vestnik (Vestnik Mos-kovskogo gosudarstvennogo universiteta lesa), 2009, no. 5(68), pp. 141–145. (in Russ.).
Anderbrant O., Löfqvist J., Högberg H.-E., Hedenstrom E.; Baldassari N., Baronio P., Kolmakova G., Lyons B., Naito T., Odinokov V., Simandl J., Supatashvili A., Tai A., Tourianov R. Entomol. Exp. Appl., 2000, vol. 95, no. 3, pp. 229–239. DOI: 10.1046/j.1570-7458.2000.00662.x.
Kikukawa T., Matsumura F., Kraemer M., Coppel H.C., Tai A. J. Chem. Ecol., 1982, vol. 8, no. 1, pp. 301–314. DOI: 10.1007/BF00984025.
Olaifa J.I., Kikukawa T., Matsumura F., Coppel H.C. Environ. Entomol., 1984, vol. 13, no. 5, pp. 1274–1277. DOI: 10.1093/ee/13.5.1274.
Olaifa J.I., Matsumura F., Kikukawa T., Coppel H.C. J. Chem. Ecol., 1988, vol. 14, no. 4, pp. 1131–1144. DOI: 10.1007/BF01019341.
Longhurst C., Baker R., Mori K. Experientia, 1980, vol. 36, no. 8, pp. 946–947. DOI: 10.1007/BF01953808.
Hedenström E., Edlund H., Wassgren A.-B., Bergström G., Anderbrant O., Östrand F., Sierpinski A., Auger-Rozenberg M.-A., Herz A., Heitland W., Varama M. J. Chem. Ecol., 2006, vol. 32, no. 11, pp. 2525–2541. DOI: 10.1007/s10886-006-9161-5.
Kraemer M.E., Coppel H.C., Matsumura F., Kikukawa T., Benoit P. J. Chem. Ecol., 1984, vol. 10, no. 7, pp. 983–995. DOI: 10.1007/BF00987507.
Bång J., Hedenström E., Anderbrant O. Anal. Lett., 2012, vol. 45, no. 9, pp. 1016–1027. DOI: 10.1080/00032719.2012.670789.
Bång J., Hedenström E., Sjödin K. J. Chem. Ecol., 2011, vol. 37, no. 1, pp. 125–133. DOI: 10.1007/s10886-010-9886-z.
Keeling C.I., Plettner E., Slessor K.N. Topics in Current Chemistry. Berlin, Heidelberg, 2004, vol. 239, pp. 133–177. DOI: 10.1007/b95452.
Pines H., Hoffman N.E., Ipatieff V.N. J. Amer. Chem. Soc., 1954, vol. 76, no. 17, pp. 4412–4416. DOI: 10.1021/ja01646a041.
Banthorpe D.V., Whittaker D. Chem. Revs., 1966, vol. 66, no. 4–5, pp. 643–656. DOI: 10.1021/cr60244a002.
Odinokov V.N., Ishmuratov G.Yu., Ladenkova I.M., Muslukhov R.R., Berg A.A., Serebryakov E.P., Tolstikov G.A. Khimiya prirodnykh soyedineniy, 1992, no. 1, pp. 117–122. (in Russ.).
Khao N.K., Mavrov M.V., Serebryakov E.P. Izv. AN SSSR. Ser. khim., 1988, no. 5, pp. 1142–1146. (in Russ.).
Tai A., Imaida M., Oda T., Watanabe H. Chem. Lett., 1978, vol. 9, no. 39, pp. 61–64. DOI: 10.1002/chin.197839325.
Ebert S., Krause N. Eur. J. Org. Chem., 2001, vol. 2001, no. 20, pp. 3831–3835. DOI: 10.1002/1099-0690(200110)2001:203.0.CO;2-2.
Krause N., Ebert S., Haubrich A. Liebigs Annalen., 1997, vol. 1997, no. 12, pp. 2409–2418. DOI: 10.1002/jlac.199719971204.
Schreiber S.L. J. Amer. Chem. Soc., 1980, vol. 102, no. 19, pp. 6163–6165. DOI: 10.1021/ja00539a041.
Petasis N.A., Lu S.-P. Tetrahedron Lett., 1995, vol. 36, no. 14, pp. 2393–2396. DOI: 10.1016/0040-4039(95)00320-C.
Lundh M., Smitt O., Hedenström E. Tetrahedron Asymmetry, 1996, vol. 7, no. 11, pp. 3277–3284. DOI: 10.1016/0957-4166(96)00428-4.
Mori K., Tamada S., Matsui M. Tetrahedron Lett., 1978, vol. 19, no. 10, pp. 901–904. DOI: 10.1016/S0040-4039(01)91431-5.
Mori K., Tamada S. Tetrahedron., 1979, vol. 35, no. 10, pp. 1279–1284. DOI: 10.1016/0040-4020(79)80054-X.
Patent 54106405A (JP). 1979.
Odinokov V.N., Akhmetova V.R., Khasanov Kh.D., Abduvakhabov A.A., Khalilov L.M., Cheskis B.A., Moiseyen-kov A.M., Tolstikov G.A. Zhurnal organicheskoy khimii, 1992, vol. 28, no. 6, pp. 1163–1172. (in Russ.).
Odinokov V.N., Akhmetova V.R., Khasanov Kh.D., Abduvakhabov A.A., Cheskis B.A., Moiseyenkov A.M., Tolstikov G.A. Dokl. AN SSSR, 1991, vol. 317, no. 3, pp. 653–657. (in Russ.).
Odinokov V.N., Akhmetova V.R., Khasanov Kh.D., Abduvakhabov A.A., Khalilov L.M., Cheskis B.A., Moiseyen-kov A.M., Tolstikov G.A. Zhurnal organicheskoy khimii, 1992, vol. 28, no. 7, pp. 1339–1345. (in Russ.).
Larcheveque M., Sanner C., Azerad R., Buisson D. Tetrahedron, 1988, vol. 44, no. 20, pp. 6407–6418. DOI: 10.1016/s0040-4020(01)89828-8.
Larcheveque M., Petit Y. Tetrahedron Lett., 1987, vol. 28, no. 18, pp. 1993–1996. DOI: 10.1016/S0040-4039(0 0)96028-3.
Kikukawa T., Imaida M., Tai A. Chem. Lett., 1982, vol. 11, no. 11, pp. 1799–1802. DOI: 10.1246/cl.1982.1799.
Kikukawa T., Imaida M., Tai A. Bull. Chem. Soc. Jpn., 1984, vol. 57, no. 7, pp. 1954–1960. DOI: 10.1246/bcsj.57.1954.
Tai A., Morimoto N.,1 Yoshikawa M., Uehara K., Sugimura T., Kikukawa T. Agric. Biol. Chem., 1990, vol. 54, no. 7, pp. 1753–1762. DOI: 10.1271/bbb1961.54.1753.
Tai A., Sugimura T., Kikukawa T., Naito C., Nishimoto Yu., Morimoto N. Biosci. Biotech. Biochem., 1992, vol. 56, no. 11, pp. 1711–1714. DOI: 10.1271/bbb.56.1711.
Ishmuratov G.Yu., Yakovleva M.P., Ishmuratova N.M., Vydrina V.A., Muslukhov R.R., Tolstikov G.A. Khimiya v usloviyakh ustoychivogo razvitiya, 2008, vol. 16, no. 6, pp. 721–725. (in Russ.).
Ishmuratov G.Yu., Yakovleva M.P., Vydrina V.A., Khasanova E.F., Muslukhov R.R., Ishmuratova N.M., Tolstikov G.A. Khimiya Rastitel'nogo Syr'ya, 2007, no. 3, pp. 23–32. (in Russ.).
Ishmuratov G.Yu., Yakovleva M.P., Ishmuratova N.M., Tolstikov A.G., Tolstikov G.A. Monoterpenoidy v khimii opticheski aktivnykh feromonov nasekomykh. [Monoterpenoids in the chemistry of optically active insect pheromones]. Moscow, 2012, 171 p. (in Russ.).
Ishmuratov G.Y., Yakovleva M.P., Valeeva E.F., Vydrina V.A., Tolstikov G.A. Russ. J. Bioorgan. Chem., 2012, vol. 38, no. 7, pp. 667–688. DOI: 10.1134/S1068162012070084.
Yakovleva M.P., Khasanova E.F., Vydrina V.A., Ishmuratova N.M., Talipov R.F., Ishmuratov G.Yu. Vestnik Bash-kirskogo universiteta, 2008, vol. 13, no. 4, pp. 891–894. (in Russ.).
Ishmuratov G.Yu., Yakovleva M.P., Ganieva V.A., Kharisov R.Ya., Gazetdinov R.R., Abulkaramova A.M., Tolstikov G.A. Khimiya prirodnykh soyedineniy, 2006, no. 1, pp. 73–76. (in Russ.).
Ishmuratov G.Yu., Yakovleva M.P., Ganieva V.A., Muslukhov R.R., Tolstikov G.A. Khimiya prirodnykh soyed-ineniy, 2005, no. 1, pp. 33–36. (in Russ.).
Ishmuratov G.Yu., Yakovleva M.P., Ganieva V.A., Amirkhanov D.V., Tolstikov G.A. Khimiya prirodnykh soyed-ineniy, 2005, no. 6, pp. 592–593. (in Russ.).
Latypova E.R., Bannova A.V., Muslukhov R.R., Shutova M.A., Talipov R.F., Ishmuratov G.Yu. Khimiya prirodnykh soyedineniy, 2010, no. 3, pp. 312–314. (in Russ.).
Yakovleva M.P., Vydrina V.A., Ishmuratova N.M., Ishmuratov G.Yu. Ekobiotekh, 2020, vol. 3, pp. 634–642. DOI: 10.31163/2618-964X-2020-3-4-634-642. (in Russ.).
Kovalenko V., Matiushenkov E. Tetrahedron: Asymmetry, 2012, vol. 23, no. 18–19, pp. 1393–1399. DOI: 10.1016/j.tetasy.2012.09.002.
Wang Z., Xu Q., Tian W., Pan X. Tetrahedron Lett., 2007, vol. 48, no. 42, pp. 7549–7551. DOI: 10.1016/j.tetlet.2007.06.123.
Patent 101007765 A (CN). 2007.
Wang Z.-K., Tian W.-S., Pan X.-F. Chin. J. Org. Chem., 2007, vol. 27, no. 7, pp. 866–869.
Patent 100548965 C (CN). 2004.
Moreira J.A., Corrêa A.G. J. Braz. Chem. Soc., 2000, vol. 11, no. 6, pp. 614–620. DOI: 10.1590/S0103-50532000000600010.
Wang S.-Y., Song P., Chin Y.-J., Loh T.-P. Chem. Asian J., 2011, vol. 6, no. 2, pp. 385–388. DOI: 10.1002/asia.201000663.
Byström S., Högberg H.-E., Norin T. Tetrahedron, 1981, vol. 37, no. 12, pp. 2249–2254. DOI: 10.1016/s0040-4020(01)97980-3.
Högberg H.-E., Hedenström E., Wassgren A.-B., Hjalmarsson M., Bergström G., Löfqvist J., Norin T. Tetrahedron, 1990, vol. 46, no. 8, pp. 3007–3018. DOI: 10.1016/S0040-4020(01)88392-7.
Hedenström E., Högberg H.-E., Wassgren A.B., Bergstroem G., Loefqvist J., Hansson B., Anderbrant O. Tetrahe-dron, 1992, vol. 48, no. 15, pp. 3139–3146. DOI: 10.1016/S0040-4020(01)92255-0.
Guoqiang L., Hjalmarsson M., Högberg H.-E., Jemstedt K., Norin T. Acta Chem. Scand., 1984, vol. 38B, no. 9, pp. 795–801. DOI: 10.3891/acta.chem.scand.38b-0795.
Itoh T., Yonekawa Y., Sato T., Fujisawa T. Tetrahedron Lett., 1986, vol. 27, no. 44, pp. 5405–5408. DOI: 10.1016/s0040-4039(00)85223-5.
Bekish A.V., Prokhorevich K.N., Kulinkovich O.G. Eur. J. Org. Chem., 2006, vol. 22, pp. 5069–5075. DOI: 10.1002/ejoc.200600481.
Zheng J.F., Lan H.Q., Yang R.F., Peng Q.L., Xiao Z.H., Tuo S.C., Hu K.Z., Xiang Y., Wei Z., Zhang Z., Huang P.Q. Helv. Chim. Acta, 2012, vol. 95, no. 10, pp. 1799–1808. DOI: 10.1002/hlca.201200341.
Patent 102167666 A (CN). 2011.
Huang P.-Q., Lan H.-Q., Zheng X., Ruan Y.-P. J. Org. Chem., 2004, vol. 69, no. 11, pp. 3964–3967. DOI: 10.1021/jo0497961.
Patent 1470490A (CN). 2004.
Copyright (c) 2024 chemistry of plant raw material

This work is licensed under a Creative Commons Attribution 4.0 International License.

This work is licensed under a Creative Commons Attribution 4.0 International License.
The authors, which are published in this journal, agree to the following conditions:
1. Authors retain the copyright to the work and transfer to the journal the right of the first publication along with the work, at the same time licensing it under the terms of the Creative Commons Attribution License, which allows others to distribute this work with the obligatory indication of the authorship of this work and a link to the original publication in this journal .
2. The authors retain the right to enter into separate, additional contractual agreements for the non-exclusive distribution of the version of the work published by this journal (for example, to place it in the university depository or to publish it in a book), with reference to the original publication in this journal.
3. Authors are allowed to post their work on the Internet (for example, in a university repository or on their personal website) before and during the review process of this journal, as this may lead to a productive discussion, as well as more links to this published work.











