OBTAINING AND INVESTIGATION OF THE BIOLOGICAL ACTIVITY OF GUANIDINE COMPLEXES WITH PECTIN POLYSACCHARIDES
UDC 541.6.69:615.01
Abstract
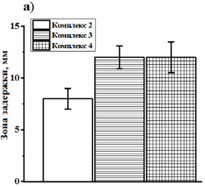
Pectin polysaccharides, due to the presence of reactive carboxyl functional groups in the structure, have a high complexing ability. This expands the scope of their practical application for the development of new biologically active substances. In this work, we propose the preparation of complex compounds based on sodium pectate and guanidine hydrochloride. By varying the molar amount of guanidine in relation to the acid groups of the polysaccharide matrix, samples were obtained that differ in the degree of substitution, the content of the amino derivative of the compound, and the inherent viscosity. The optimal ratio of the starting compounds was determined, at which the limiting amount of guanidine is bound to -COO- groups of pectin macromolecules. The structure of the resulting complexes was studied by FTIR spectroscopy. It has been established that the interaction of guanidine with polysaccharide carboxyls is accompanied by a change in the intensity of the absorption bands corresponding to the ionized acid group. Also, the binding of guanidine to the polysaccharide matrix through ionic bonds has been proven by carrying out the hydrolytic cleavage of the complex compound in an acidic medium. It is shown that with an increase in the hydrolysis time from 3 to 24 h, a gradual decrease in the initial degree of substitution of the complex compound is observed. The antimicrobial properties of polymer complexes with different characteristics against Staphylococcus aureus and Escherichia coli were studied.
Downloads
Metrics
References
Qian L., Guan Y., He B., Xiao H. Polymer, 2008, vol. 49(10), pp. 2471–2475. DOI: 10.1016/j.polymer.2008.03.042.
Bromberg L., Hatton T.A. Polymer, 2007, vol. 48(26), pp. 7490–7498. DOI: 10.1016/j.polymer.2007.10.040.
Stel'makh S.A., Bazaron L.U., Mognonov D.M. Zhurnal prikladnoy khimii, 2010, vol. 83, no. 2, pp. 244–246. (in Russ.).
Grigor'yeva M.N., Stel'makh S.A., Astakhova S.A., Tsenter I.M., Bazaron L.U., Batoyev V.B., Mognonov D.M. Khimiko-farmatsevticheskiy zhurnal, 2015, vol. 49, no. 2, pp. 29–33. DOI: 10.30906/0023-1134-2015-49-2-29-33. (in Russ.).
Wender P.A., Galliher W.C., Goun E.A., Jones L.R., Pillow T.H. Advanced Drug Delivery Reviews, 2008, vol. 60(4-5), pp. 452–472. DOI: 10.1016/j.addr.2007.10.016.
Salama H.E., Abdel Aziz M.S. International Journal of Biological Macromolecules, 2020, vol. 165, pp. 1187–1197. DOI: 10.1016/j.ijbiomac.2020.09.25.
Zhao X., He J., Zhan Y. Polymer Journal, 2009, vol. 41(12), pp. 1030–1035. DOI: 10.1295/polymj.pj2009087.
Pan Y., Zhao X., Li X., Cai P. Polymers, 2019, vol. 11(11), article 1846. DOI: 10.3390/polym11111846.
Mi X., Albukhari S.M., Heldt C.L., Heiden P.A. Carbohydrate Research, 2020, article 108153. DOI: 10.1016/j.carres.2020.108153.
Liu K., Xu Y., Lin X., Chen L., Huang L., Cao S., Li J. Carbohydrate Polymers, 2014, vol. 110, pp. 382–387. DOI: 10.1016/j.carbpol.2014.03.086.
Akhmedov O.R., Shomurotov Sh.A., Turaev A.S. Khimiya Rastitel'nogo Syr'ya, 2022, no. 3, pp. 81–90. DOI: 10.14258/jcprm.20220310882. (in Russ.).
Akhmedov O.R., Shomurotov Sh.A., Turaev A.S., Sidorenko A.V. Razrabotka i registratsiya lekarstvennykh sredstv, 2022, vol. 11, no. 2, pp. 38–45. DOI: 10.33380/2305-2066-2022-11-2-38-45. (in Russ.).
Bodyakina I.M., Bagryantsev V.A., Kotov V.V., Lukin A.L. Vestnik VGU, seriya: Khimiya. Biologiya. Farmatsiya, 2012, no. 2, pp. 9–14. (in Russ.).
Minzanova S.T., Mironov V.F., Mironova L.G., Nizameev I.R., Kholin K.V., Voloshina A.D., Kulik N.V., Naza-rov N.G., Milyukov V. A. Chemistry of Natural Compounds, 2016, vol. 52, pp. 26–31. DOI: 10.1007/s10600-016-1539-1.
Shatalov D.O. Razrabotka i standartizatsiya metodov kontrolya kachestva, razvetvlennogo oligogeksametilenguanidin gidrokhlorida: diss. ... kand. farm. nauk. [Development and standardization of quality control methods for branched ol-igohexamethyleneguanidine hydrochloride: diss. ... cand. farm. sciences]. Moscow, 2015, 137 p. (in Russ.).
Muhidinov Z.K., Fishman M.L., Avloev K.K., Norova M.T., Nasriddinov A.S., Khalikov D.K. Polymer Science Series A, 2010, vol. 52(12), pp. 1257–1263. DOI: 10.1134/s0965545x10120035.
Akhmedov O.R., Shomurotov Sh.A., Turaev A.S. Khimiya Rastitel'nogo Syr'ya, 2021, no. 3, pp. 73–82. DOI: 10.14258/jcprm.2021038705. (in Russ.).
Navashin S.M., Fomina I.P. Ratsional'naya antibiotikoterapiya. [Rational antibiotic therapy]. Moscow, 1982, 496 p. (in Russ.).
Afinogenov G.Ye., Panarin Ye.F. Antimikrobnyye polimery. [Antimicrobial polymers]. St. Petersburg, 1993, 264 p. (in Russ.).
Gilbert P., Moore L.E. Journal of Applied Microbiology, 2005, vol. 99, pp. 703–715. DOI: 10.1111/j.1365-2672.2005.02664.x
Copyright (c) 2023 chemistry of plant raw material

This work is licensed under a Creative Commons Attribution 4.0 International License.

This work is licensed under a Creative Commons Attribution 4.0 International License.
The authors, which are published in this journal, agree to the following conditions:
1. Authors retain the copyright to the work and transfer to the journal the right of the first publication along with the work, at the same time licensing it under the terms of the Creative Commons Attribution License, which allows others to distribute this work with the obligatory indication of the authorship of this work and a link to the original publication in this journal .
2. The authors retain the right to enter into separate, additional contractual agreements for the non-exclusive distribution of the version of the work published by this journal (for example, to place it in the university depository or to publish it in a book), with reference to the original publication in this journal.
3. Authors are allowed to post their work on the Internet (for example, in a university repository or on their personal website) before and during the review process of this journal, as this may lead to a productive discussion, as well as more links to this published work.











