DEVELOPMENT OF APPROACHES TO MOLECULAR MODELING OF THE INTERACTION OF BIOLOGICALLY ACTIVE COMPONENTS OF HUMIC SUBSTANCES WITH BETA-LACTAMASES ON THE EXAMPLE OF HUMIC-LIKE LOW-MOLECULAR WEIGHT ANALOGUES
UDK 577(11+151+181)
Abstract
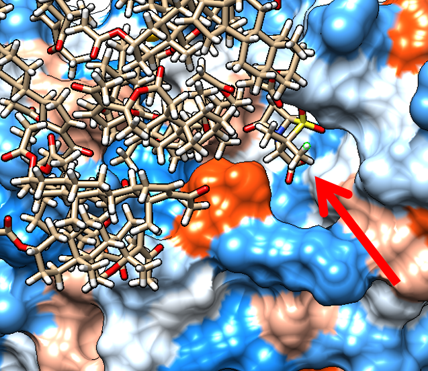
β-Lactamase inhibitors are used to protect betalactam antibiotics from the action of antibiotic resistant bacteria. However, due to the emergence of resistance to β-lactam inhibitors, the search for new non-betalactam inhibitors is an urgent task. In this work, natural humic substances (HS) are considered as a new source of beta-lactamase inhibitors. We previously reported on the ability of coal humic acids and their narrow fractions to inhibit TEM-1 β-lactamases. The purpose of this work was to study the mechanism of interaction between the molecular components of humic substances using molecular modeling. The selection of humic-like molecules was carried out using the dereplication method based on the known inhibitory activity of the HS fraction and its molecular composition as determined by high-resolution mass spectrometry. Modeling of the interaction of selected molecules with beta-lactamase protein was carried out using docking and molecular dynamics methods using Chimera v.1.15 software and Amber14. As a result of dereplication, 156 unique structures were identified, of which three were selected. The modeling results showed that the most likely mechanism of interaction of HS with beta-lactamase is non-competitive inhibition as a result of binding to the allosteric site of the protein. In addition, nonspecific aggregation on the protein surface is possible, which also explains the synergistic effect of HS with respect to sulbactam, which might be sterically blocked in the active site of betalactamase. The prospects of using the dereplication method for studying the molecular mechanisms of the biological activity of humic substances are shown.
Downloads
Metrics
References
Hamad B. Nature reviews Drug discovery, 2010, vol. 9, no. 9, pp. 675. DOI: 10.1038/nrd3267.
WHO. New report calls for urgent action to avert antimicrobial resistance crisis. // World Health Organization website. https://www. who. int/news-room/detail/29-04-2019-new-report-calls-for-urgent-action to avert-antimicrobial-resistance-crisis. Accessed. 2023. DOI: 10.1007/s12668-019-00658-4.
Blair J.M., Webber M.A., Baylay A.J., Ogbolu D.O., Piddock L.J. Nature reviews microbiology, 2015, vol. 13, no. 1, pp. 42–51. DOI: 10.1038/nrmicro3380.
Hall B.G., Barlow M. Journal of Antimicrobial Chemotherapy, 2005, vol. 55, no. 6, pp. 1050–1051. DOI: 10.1093/jac/dki130.
Biondi S., Long S., Panunzio M., L. Qin W. Current medicinal chemistry, 2011, vol. 18, no. 27, pp. 4223–4236. DOI: 10.2174/092986711797189655.
Docquier J.D., Mangani S. Drug Resistance Updates, 2018, vol. 36, pp. 13–29. DOI: 10.1016/j.drup.2017.11.002.
Strynadka N.C., Jensen S.E., Alzari P.M., James M.N. Nature structural biology, 1996, vol. 3, no. 3, pp. 290–297. DOI: 10.1038/nsb0396-290.
Roccatano D., Sbardella G., Aschi M., Amicosante G., Bossa C., Nola A.D., Mazza F. Journal of computer-aided molecular design, 2005, vol. 19, pp. 329–340. DOI: 10.1007/s10822-005-7003-0.
Jelsch C., Mourey L., Masson J.M., Samama J.P. Proteins: Structure, Function, and Bioinformatics, 1993, vol. 16, no. 4, pp. 364–383. DOI: 10.1002/prot.340160406.
Livermore D.M. Journal of Antimicrobial Chemotherapy, 1993, vol. 31, pp. 9–21. DOI: 10.1093/jac/31.suppl_A.9.
Drawz S.M., Bonomo R.A. Clinical microbiology reviews, 2010, vol. 23, no. 1, pp. 160-201. DOI: 10.1128/cmr.00037-09.
Orlov A.A., Zherebker A., Eletskaya A.A., Chernikov V.S., Kozlovskaya L.I., Zhernov Y.V., Kostyukevich Y., Palyulin V.A., Nikolaev E.N., Osolodkin D.I., Perminova I.V. Scientific Reports, 2019, vol. 9, no. 1, pp. 12066. DOI: 10.1038/s41598-019-48000-y.
Piccolo A. Advances in Agronomy, 2002, pp. 57–134. DOI: 10.1016/S0065-2113(02)75003-7.
Kleinhempel D. Archives of Agronomy and Soil Science, 1970, vol. 14, no. 1, pp. 3–14. DOI: 10.1080/03650347009412655.
Mikhnevich T.A., Vyatkina A.V., Grigorenko V.G., Rubtsova M.Y., Rukhovich G.D., Letarova M.A., Kravtsova D.S., Vladimirov S.A., Orlov A.A., Nikolaev E.N., Zherebker A. ACS omega, 2021, vol. 6, no. 37, pp. 23873–23883. DOI: 10.1021/acsomega.1c02841.
Zhernov Y.V., Kremb S., Helfer M., Schindler M., Harir M., Mueller C., Hertkorn N., Avvakumova N.P., Konstantinov A.I., Brack-Werner R., Schmitt-Kopplin P. New Journal of Chemistry, 2017, vol. 41, no. 1, pp. 212–224. DOI: 10.1039/C6NJ00960C.
Zhernov Y.V., Konstantinov A.I., Zherebker A., Nikolaev E., Orlov A., Savinykh M.I., Kornilaeva G.V., Karamov E.V., Perminova I.V. Environmental Research, 2021, vol. 193, pp. 110312. DOI: 10.1016/j.envres.2020.110312.
Klöcking H.P. In Recent Developments in Toxicology: Trends, Methods and Problems: Proceedings of the European Societies of Toxicology Meeting Held in Leipzig. 1991, pp. 166–169. DOI: 10.1007/978-3-642-74936-0_33.
Zeck-Kapp G., Nauck M., Riede U.N., Block L., Freudenberg N., Seubert B. Verh. Dtsch. Ges. Path., 1991, vol. 75, pp. 504.
Neyts J., Snoeck R., Wutzler P., Cushman M., Klöcking R., Helbig B., Wang P., De Clercq E. Antiviral Chemistry and Chemotherapy, 1992, vol. 3, no. 4, pp. 215–222. DOI: 10.1177/095632029200300404.
Orlov A.A., Zherebker A., Eletskaya A.A., Chernikov V.S., Kozlovskaya L.I., Zhernov Y.V., Kostyukevich Y., Palyulin V.A., Nikolaev E.N., Osolodkin D.I., Perminova I.V. Scientific Reports, 2019, vol. 9, no. 1, pp. 12066. DOI: 10.1038/s41598-019-48000-y.
Mohimani H., Gurevich A., Shlemov A., Mikheenko A., Korobeynikov A., Cao L., Shcherbin E., Nothias L.F., Dorrestein P.C., Pevzner P.A. Nature communications, 2018, vol. 9, no. 1, pp. 4035. DOI: 10.1038/s41467-018-06082-8.
Cornell W.D., Cieplak P., Bayly C.I., Gould I.R., Merz K.M., Ferguson D.M., Spellmeyer D.C., Fox T., Caldwell J.W., Kollman P.A. Journal of the American Chemical Society, 1995, vol. 117, no. 19, pp. 5179–5197. DOI: 10.1021/ja00124a002.
Maier J.A., Martinez C., Kasavajhala K., Wickstrom L., Hauser K.E., Simmerling C. Journal of chemical theory and computation, 2015, vol. 11, no. 8, pp. 3696–3713. DOI: 10.1021/acs.jctc.5b00255
Pettersen E.F., Goddard T.D., Huang C.C., Couch G.S., Greenblatt D.M., Meng E.C., Ferrin T.E. Journal of computational chemistry, 2004, vol. 25, no. 13. Pp 1605–1612. DOI: 10.1002/jcc.20084.
Wang J., Wolf R.M., Caldwell J.W., Kollman P.A., Case D.A. Journal of computational chemistry, 2004, vol. 25, no. 9, pp. 1157–1174. DOI: 10.1002/jcc.20035.
Petrov D., Tunega D., Gerzabek M.H., Oostenbrink C. Environmental Science & Technology, 2017, vol. 51, no. 10, pp. 5414–5424. DOI: 10.1021/acs.est.7b00266.
Ai Y., Zhao C., Sun L., Wang X., Liang L. Science of the Total Environment, 2020, vol. 702, pp. 135072. DOI: 10.1016/j.scitotenv.2019.135072.
Bowman G.R., Geissler P.L. Proceedings of the National Academy of Sciences, 2012, vol. 109, no. 29, pp. 11681–11686. DOI: 10.1073/pnas.1209309109.
Avci F.G., Altinisik F.E., Vardar U.D., Ozkirimli O.E., Sariyar A.B. Journal of enzyme inhibition and medicinal chemistry, 2016, vol. 31, no. 3, pp. 33–40. DOI: 10.1016/j.jmb.2003.12.068.
Avci F.G., Altinisik F.E., Karacan I., Karagoz D.S., Ersahin S., Eren A., Sayar N.A., Ulu D.V., Ozkirimli E., Akbulut B.S. Journal of Molecular Graphics and Modelling, 2018, vol. 84, pp. 125–133. DOI: 10.1016/j.jmgm.2018.06.007.
Horn J.R., Shoichet B.K. Journal of molecular biology, 2004, vol. 336, no. 5, pp. 1283–1291. DOI: 10.1080/14756366.2016.1201813.
Copyright (c) 2024 chemistry of plant raw material

This work is licensed under a Creative Commons Attribution 4.0 International License.

This work is licensed under a Creative Commons Attribution 4.0 International License.
The authors, which are published in this journal, agree to the following conditions:
1. Authors retain the copyright to the work and transfer to the journal the right of the first publication along with the work, at the same time licensing it under the terms of the Creative Commons Attribution License, which allows others to distribute this work with the obligatory indication of the authorship of this work and a link to the original publication in this journal .
2. The authors retain the right to enter into separate, additional contractual agreements for the non-exclusive distribution of the version of the work published by this journal (for example, to place it in the university depository or to publish it in a book), with reference to the original publication in this journal.
3. Authors are allowed to post their work on the Internet (for example, in a university repository or on their personal website) before and during the review process of this journal, as this may lead to a productive discussion, as well as more links to this published work.











