PHYTOECDYSTEROIDS OF ROOT SILENE FRIVALDSZKYANA HAMPE
Abstract
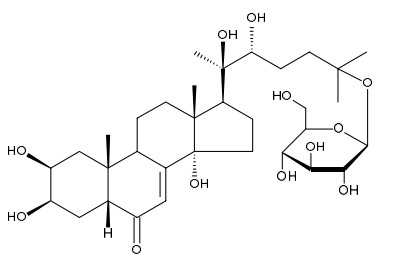
Ecdysteroids were isolated from roots of Silene frivaldszkyana (Caryophyllaceae) introduced into culture in Western Siberia: 20-hydroxyecdysone, polypodine B, integristerone A, 2-deoxy-20-hydroxyecdysone, 2-deoxyecdysone, 20,26-dihydroxyecdysone, 26-hydroxypolypodine B, 26-hydroxyintegristerone A, 20-hydroxyecdysone-25-β-D-glucoside. Structures are installed using NMR, mass spectroscopy, HPLC. The presence of 26-hydroxyintegristerone A in the roots established by comparative HPLC analysis with compound isolated from overground part of plants.
It is shown that this Silene species synthesizes characteristic for this genus of ecdysteroids: 2-deoxy-20-hydroxyecdysone, 2-deoxyecdysone, 20-hydroxyecdysone, polypodine B, integristerone A, and is a rich source of their 26-hydroxy derivatives. Among the common ecdysteroids of the genus Silene 20-hydroxyecdysone-25-β-D-glycoside was isolated for the first from roots of S. frivaldszkyana.
Comparative analysis of ecdysteroids in aboveground and underground parts showed that the roots contain a richer composition of them that is likely so that the roots of perennial plants accumulate these compounds. And 20-hydroxyecdysone-25-β-D-glycoside is a spare substance of major component 20-hydroxyecdysone.
Downloads
Metrics
References
Зибарева Л.Н., Еремина В.И., Иванова Н.А. Новые экдистероидоносные виды рода Silene L. и динамика содер-жания в них экдистерона // Растительные ресурсы. 1997. Т. 33, вып. 3. С. 73–76.
Zibareva L. Distribution and levels of phytoecdysteroids in plants of genus Silene during development // Archives
of insect biochemistry and physiology. 2000. Vol. 43. Pp. 1–8.
Zibareva L., Yeriomina V. I., Munkhjargal N., Girault J.-P., Dinan L.,. Lafont R. The Phytoecdysteroid Profiles of 7 Species of Silene (Caryophyllaceae) // Archives of insect biochemistry and physiology. 2009. Vol. 72. N4. Pp. 234–248.
Патент № 2445110 (РФ). Способ получения 26-интегристерона А из растительного сырья / Л.Н. Зибарева, О.В. Волкова / 07.12.2010.
Ecdybase [Electronic resource]. URL: http://www.ecdybase.org
Зибарева Л.Н., Волкова О.В., Лафон Р. Виды рода Silene – продуценты 26-гидроксиэкдистероидов // Теоретиче-ская и прикладная экология. 2012. №1. С. 66–72.
Nishimoto N., Shiobara Y., Inoue S., Fujino M. Three ecdysteroid glycosides from Pfaffia iresinoides // Phytochemis-try. 1988. Vol. 27. Рp. 1665–1668.
Girault J.-P., Bathori M., Varga E., Szendrei K., Lafont R. Isolation and identification of new ecdysteroids from the Caryophyllaceae // J. of Natural Products. 1990. Vol. 53. Pp. 279–293.

This work is licensed under a Creative Commons Attribution 4.0 International License.
The authors, which are published in this journal, agree to the following conditions:
1. Authors retain the copyright to the work and transfer to the journal the right of the first publication along with the work, at the same time licensing it under the terms of the Creative Commons Attribution License, which allows others to distribute this work with the obligatory indication of the authorship of this work and a link to the original publication in this journal .
2. The authors retain the right to enter into separate, additional contractual agreements for the non-exclusive distribution of the version of the work published by this journal (for example, to place it in the university depository or to publish it in a book), with reference to the original publication in this journal.
3. Authors are allowed to post their work on the Internet (for example, in a university repository or on their personal website) before and during the review process of this journal, as this may lead to a productive discussion, as well as more links to this published work.











