HISTOCHEMICAL STUDY XYLEM CELLS HAVE IRIS SIBIRICA L. IN CULTURE IN VITRO
Abstract
This paper presents the data content of the lignin from annual plants-regenerants Iris sibirica is comparable to the content of a 6-year-old intact plants. With the aim of identifying conditions of accelerated lignification studied the structure of the shoots of Iris sibirica , grown on artificial nutrient media, using the methods of histochemistry.
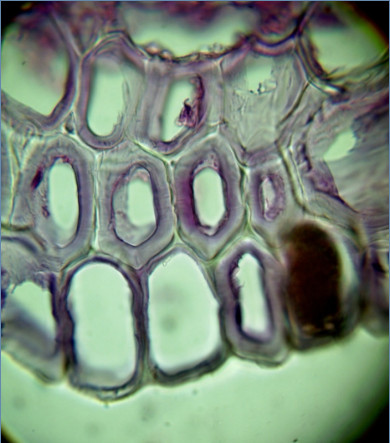
Peculiarities of the formation of the xylem from Iris sibirica on artificial nutrient media. Very quickly regenerants developed a complex system consisting of conducting bundles containing sieve tubes, vessels and tracheids, and as a network of gidrozit. Hydrocity Iris sibirica in its structure is tracheids with significiance the swelling, but, in contrast to tracheids and vessels of xylem (they are formed on the basis of procube or cambium – lateral special primary or secondary meristem), hydrocity differentiated from the cells of permanent tissues (like phellogen), which probably at the time of differentiation possessed meristematic activity. From Iris sibirica hydrocity thick layer envelops the conductive beam and accompanied him along the escape at a certain height. Due to the formation of the thick fabric of significiance tracheal elements, young regenerants Iris sibirica was high lignin content.
The study of the differentiation of xylem elements in the laboratory can serve as a model for our understanding of the processes of formation of wood.
Downloads
Metrics
References
Turner J.A., Buongiorno J., Maplesden F., Zhu S., Bates S., Li R. World Wood Industries Outlook // Forest Research Bulletin. 2006. Vol. 230. Pp. 2005–2030. I
Turner S., Gallois P., Brown D. Tracheary Element Differentiation // Annu. Rev. Plant Biol. 2007. Vol. 58. Pp. 407–433.
Fukuda H. Plant tracheary elements // Encyclopedia of Life Sciences. 2010. Pp. 1–5.
Bollhöner B., Prestele J., Tuominen H. Xylem cell death: emerging understanding of regulation and function // Journal of Experimental Botany. 2012. Vol. 63. N3. Pp. 1081–1094.
Oda Y., Fukuda H. Secondary cell wall patterning during xylem differentiation // Current Opinion in Plant Biology. 2012. Vol. 15. N1. Pp. 38–44.
Антонова Г.Ф., Железниченко Т.В., Стасова В.В. Лигнификация каллуса сосны обыкновенной как реакция на условия культивирования и состав питательной среды // Cибирский лесной журнал. 2014. №6. С. 46–59.
Preeti Dahiya. Role of death in providing lifeline to plants // Trends in Plant Science. 2003. Vol. 8. Pp. 462–465.
Pesquet E., Ranocha P., Legay S., Digonnet C., Barbier O., Pichon M., Goffner D. Novel Markers of Xylogenesis in Zinnia Are Differentially Regulated by Auxin and Cytokinin1,[W] // Plant physiol. 2005. Vol. 139. Pp.1821–1839.
Oda Y., Hasezawa S. Review Cytoskeletal organization during xylem cell differentiation // Plant Tissue Cult. 2006. Vol. 119. N3. Pp. 167–177.
Pyo H., Demura T., Fukuda H. Tere; a novel cis-element responsible for a coordinated expression of genes related to programmed cell death and secondary wall formation during differentiation of tracheary elements // Plant J. 2007. Vol. 51. N6. Pp. 955–965.
Ibañes M., Fàbregas N., Chory J., Caño-Delgado A.I. Brassinosteroid signaling and auxin transport are required to es-tablish the periodic pattern of Arabidopsis shoot vascular bundles // Proc Natl Acad Sci USA. 2009. Vol. 106. N32. Pp. 13630–13635.
Kwon S.I., Cho H.J., Park O.K. Role of Arabidopsis RabG3b and autophagy in tracheary element differentiation // Au-tophagy. 2010. Vol. 6. N8. Pp. 1187–1189
Kwon S.I., Cho H.J., Jung J.H., Yoshimoto K., Shirasu K., Park O.K. The Rab GTPase RabG3b functions in autophagy and contributes to tracheary element differentiation in Arabidopsis. // Plant J. 2010. Vol. 64. N1. Pp. 151–164.
Fàbregas N., Ibañes M., Caño-Delgado A.I. A systems biology approach to dissect the contribution of brassinosteroid and auxin hormones to vascular patterning in the shoot of Arabidopsis thaliana // Plant Signal Behav. 2010. Vol. 5. N7. Pp. 903–906.
Milioni D., Sado P.E., Stacey N.J., Roberts K., McCann M.C. Early gene expression associated with the commitment and differentiation of a plant tracheary element is revealed by cDNA-amplified fragment length polymorphism analysis // Plant Cell. 2002. Vol. 14. N11. Pp. 2813–2824.
Demura T., Tashiro G., Horiguchi G., Kishimoto N., Kubo M., Matsuoka N., Minami A., Nagata-Hiwatashi M., Nakamura K., Okamura Y., Sassa M., Suzuki S., Yazaki J., Kikuchi S., Fukuda H. Visualization by comprehensive microarray analysis of gene expression programs during transdifferentiation of mesophyll cells into xylem cells // Proceedings of the National Academy of Sciences of the United States of America, 2002. Vol. 99. N24. Pp. 15794–15799.
Möller R., Koch G., Nanayakkara B., Schmitt U. Lignification in cell cultures of Pinus radiata: activities of enzymes and lignin topochemistry // Tree Physiology. 2005. Vol. 26. N2. Pp. 201–210.
Kubo M., Udagawa M., Nishikubo N., Horiguchi G., Yamaguchi M., Ito J., Mimura T., Fukuda H., Demura T. Transcription switches for protoxylem and me taxylem vessel formation // Genes Dev. 2005. Vol. 19. Pp. 1855–1860.
Devillard C., Walter C. Formation of plant tracheary elements in vitro – a review // New Zealand Journal of Forestry Science. 2014. Vol. 44. Pp. 2–14.
Fukuda H. Xylogenesis: initiation, progression, and cell death // Annual Review of Plant Physiology and Plant Molecular Biology. 1996. Vol. 47. N1. Pp. 299–325.
McCann M.C. Tracheary element formation: Building up to a dead end // Trends in Plant Science. 1997. Vol. 2. N9. Pp. 333–338.
Denton D., Nicolson S., Kumar S. Cell death by autophagy: facts and apparent artefacts // Cell Death and Differentiation. 2012. Vol. 19. N1. Pp. 87–95.
Kuriyama H., Fukuda H. Developmental programmed cell death in plants // Current Opinion in Plant Biology. 2002. Vol. 5. N6. Pp. 568–573.
McCabe P.F., Leaver C.J. Programmed cell death in cell cultures // Plant Molecular Biology. 2000. Vol. 44. N3. Pp. 359–368.
Fukuda, H. Signals that control plant vascular cell differentiation // Nature Reviews Molecular Cell Biology. 2004. Vol. 5. N5. Pp. 379–391.
Kohlenbach H.W., Schöpke C. Cytodifferentiation to tracheary elements from isolated mesophyll protoplasts of Zinnia elegans // Naturwissenschaften. 1981. Vol. 68. Pp. 576–577.
Roberts A.V., Walker S., Horan I., Smith E.F., Mottley J. The effect of growth retardants, humidity and lighting at Stage III on Stage IV of micropropagation inchrysanthemum and rose // Acta Hortic. 1992. Vol. 319. Рp. 153–158.
Lacayo C.I., Malkin A.J., Holman H.Y.N., Chen L., Ding S.Y., Hwang M.S., Thelen M.P. Imaging cell wall architecture in single Zinnia elegans tracheary elements // Plant Physiology. 2010. Vol. 154. N1. Pp. 121–133.
Höfte H. Plant cell biology: how to pattern a wall // Current Biology. 2010. Vol. 20. N1. Pp. 450–452.
Базарнова Н.Г., Ильичёва Т.Н., Тихомирова Л.И., Синицына А.А. Скрининг химического состава и биологиче-ской активности Iris sibirica L. сорт Cambridge // Химия растительного сырья. 2016. №3. С. 49–57.
Тихомирова Л.И., Ильичёва Т.Н., Базарнова Н.Г., Сысоева А.В. Способ получения лекарственного раститель-ного сырья лапчатки белой (Potentilla alba L.) в условиях гидропоники // Химия растительного сырья. 2016. №3. C. 59–66.
Базарнова Н.Г., Тихомирова Л.И., Фролова Н.С., Микушина И.В. Выделение и анализ экстрактивных веществ лапчатки белой (Potentilla alba L.), выращенной в различных условиях // Химия растительного сырья. 2016. №1. С. 43–51.
Оболенская А.В. Лабораторные работы по химии древесины и целлюлозы: учебное пособие. М., 1991. 320 с.
Музычкина Р.А., Корулькин Д.Ю., Абилов Ж.А. Технология производства и анализ фитопрепаратов. Алматы, 2011. 360 с.
Калинин Ф.Л., Сарнацкая В.В., Полищук В.Е. Методы культуры в физиологии и биохимии растений. Киев, 1980. 488 с.
Murashige T., Skoog F. A Revised Medium for Rapid Growth and Bioassaya with Tobacco Tissue cultures // Physiol. Plant. 1962. Vol. 15. N4. Рp. 473.
Барыкина Р.П., Веселова Т.Д., Девятов А.Г. Справочник по ботанической микротехнике. Основы и методы. М., 2004. 312 с.
Дженсен У. Ботаническая гистохимия. М., 1965. 377 с.
Клеточная оболочка растительной клетки. [Электронный ресурс]. URL: http://www.activestudy.info/kletochnaya- obolochka-rastitelnoj-kletki/.
Шанидзе М.А. Анатомический анализ вегетативных органов грузинских представителей рода Iris как материал для познания филогенеза рода : автореф. дис. … канд. биол. наук. Тбилиси, 1955. 25 с.
Эсау К. Анатомия растений. М., 1969. 564 с.
Чурикова О.А. Некоторые закономерности морфогенеза in vitro // Биотехнология как инструмент сохранения биоразнообразия растительного мира. Сборник статей по материалам II Всероссийской научно-практической конференции. Волгоград, 2008. С. 276–282.
Раздорский В.Ф. Архитектоника растений. М., 1955. 402 с.
Александров В.Г. Анатомия растений. М., 1966. 431 с.
Roberts L.W. The initiation of xylem differentiation // Bot. Rev. 1969. Vol. 35. N3. Pp. 201–250.
Wagner A., Donaldson L., Ralph J. Lignification and Lignin Manipulations in Conifers // Advances in Botanical Research. 2012. Vol. 61. Pp. 37–76.
Roberts K., McCann M.C. Xylogenesis: The birth of a corpse // Current Opinion in Plant Biology. 2000. Vol. 3. N6. Pp. 517–522.

This work is licensed under a Creative Commons Attribution 4.0 International License.
The authors, which are published in this journal, agree to the following conditions:
1. Authors retain the copyright to the work and transfer to the journal the right of the first publication along with the work, at the same time licensing it under the terms of the Creative Commons Attribution License, which allows others to distribute this work with the obligatory indication of the authorship of this work and a link to the original publication in this journal .
2. The authors retain the right to enter into separate, additional contractual agreements for the non-exclusive distribution of the version of the work published by this journal (for example, to place it in the university depository or to publish it in a book), with reference to the original publication in this journal.
3. Authors are allowed to post their work on the Internet (for example, in a university repository or on their personal website) before and during the review process of this journal, as this may lead to a productive discussion, as well as more links to this published work.











