INVESTIGATION AND OPTIMIZATION OF COMPLEXATION OF COBALT IONS WITH DIHYDROQUERCETIN IN AQUEOUS SOLUTIONS
Abstract
We continue to study the reactions of complexation of biogenic metal ions with flavonoid dihydroquercetin (DHQ). The interaction of Co2+ ions with DHQ in aqueous solutions has been studied.
It is established that complexes with different stoichiometry are formed at different pH values of the solution. Changing the pH of the solution from 6.0 to 7.0 leads to the formation of compounds 1–3 with a metal: flavonoid ligand (Met: L) ratio from 1: 2 at pH 6.0 (1) via 2: 3 at pH 6.4 -6.7 (2) to 1: 1 at pH 6.8-7.0 (3).
Using the method of thermogravimetry with elemental analysis data, the most probable composition of compounds was established with the determination of the amount of bound water: [CoL2(H2O)4] for 1, [Co2L3(OH)(H2O)4] for 2 and [CoL(OH)(H2O)2] for 3.
The conditions for the complexation of Co2+ ions with dihydroquercetin in aqueous solutions to form compound 2, optimized for the yield of the product, are proposed. pH of the solution is 6.7, the reaction time is 15 minutes, the temperature of the reaction solution is 90 °C, molar ratio of the initial reagents DHQ:Co2+ is 1: 1.5, initial concentrations are 0,020 M DHQ and 0,030 M Co2+, the source of Co2+ is CoSO4∙7H2O. The yield of the product is 81.8 %.
Downloads
Metrics
References
Trofimova N.N., Stolpovskaya E.V., Babkin V.A., Fedorov S.V., Kalabin G.A., Goryainov S.V., Zolotarev E.E., Saf-ronov A.Yu., Kashevskii A.V., Zhitov R.G. Russian Journal of Bioorganic Chemistry, 2015, vol. 41, no. 7, pp. 745–752. DOI: 10.1134/S1068162015070146.
Stolpovskaya Ye.V., Trofimova N.N, Babkin V.A. Khimiya i tekhnologiya rastitel'nykh veshchestv: materialy KH Vse-rossiyskoy nauchnoy konferentsii i shkoly molodykh uchenykh. [Chemistry and technology of plant substances: materials of the X All-Russian Scientific Conference and School of Young Scientists]. Kazan, 2017, pp. 278–279. (in Russ.).
Stolpovskaya E.V., Trofimova N.N., Babkin V.A. Russian Journal of Bioorganic Chemistry, 2017, vol. 43, no. 7, pp. 52–56. DOI: 10.1134/S1068162017070160.
Trofimova N.N., Babkin V.A., Kiselev O.I. Izvestiya Akademii nauk. Seriya khimicheskaya, 2015, no. 6, pp. 1430–1436. (in Russ.).
Patent 2637440 (RU). 2017. (in Russ.).
Babkin V.A., Ostroukhova L.A., Trofimova N.N. Biomassa listvennitsy: ot khimicheskogo sostava do innovatsion-nykh produktov. [Larch biomass: from chemical composition to innovative products]. Novosibirsk, 2011, 236 p. (in Russ.).
Panchenko L.F., Mayev I.V., Gurevich K.G. Klinicheskaya biokhimiya mikroelementov. [Clinical biochemistry of micro-elements]. Moscow, 2004, 368 p. (in Russ.).
Ayzikovich I.V., Ayzikovich B.I., Antonov A.R., Yelovskiy A.A., Ustinov D.V. Vestnik NGU. Seriya: Biologiya, klinicheskaya meditsina, 2010, vol. 8, no. 4, pp. 171–177. (in Russ.).
Korochkina Ye.A. Genetika i razvedeniye zhivotnykh, 2016, no. 3, pp. 69–73. (in Russ.).
Filippova V.A., Lysenkova A.V. Problemy zdorov'ya i ekologii, 2013, no. 4(38), pp. 72–78. (in Russ.).
Rustembekova S.A., Ametov A.S., Tliashinova A.M. Russkiy meditsinskiy zhurnal. Endokrinologiya, 2008, vol. 16, no. 16, pp. 1078–1081. (in Russ.).
Agadzhanyan N.A., Skal'nyy A.V., Detkov V.YU. Ekologiya cheloveka, 2013, no. 11, pp. 3–12. (in Russ.).
Bakhtina G.G., Len'ko O.A., Sukhanova S.Ye. Patologiya krovoobrashcheniya i kardiokhirurgiya, 2007, no. 4, pp. 82–89. (in Russ.).
Loyko O.P., Mauletova R.M., Mashentseva A.A., Khalitova A.I., Tuleuov B.I. I Mezhdunarodnaya Rossiysko-Kazakhstanskaya konferentsiya po khimii i khimicheskoy tekhnologii. [I International Russian-Kazakhstan Conference on Chemistry and Chemical Technologyъ. Tomsk, 2011. С. 313–316. (in Russ.).
Bravo A., Anacona J.R. Transition Metal Chemistry, 2001, vol. 26, pp. 20–23.
Satterfield M., Brodbelt J.S. Analytical Chemistry, 2000, vol. 72, no. 24, pp. 5898–5906.
Satterfield M., Brodbelt J.S. J. Am. Soc. Mass Spectrom,. 2001, vol. 12, pp. 537–549.
Pikulski M., Brodbelt J.S. J. Am. Soc. Mass Spectrom., 2003, vol. 14, pp. 1437–1453. DOI: 10.1016/j.jasms.2003.07.002.
Pikulski M., Wilson J.J., Aguilar A., Brodbelt J.S. Analytical Chemistry, 2006, vol. 78, no. 24, pp. 8512–8517. DOI: 10.1021 / ac061472k.
Pikulski M., Aguilar A., Brodbelt J.S. J. Am. Soc. Mass Spectrom., 2007, vol. 18, pp. 422–431. DOI: 10.1016 / j.jasms.2006.10.011.
Davis D.D., Brodbelt J.S. J. Am. Soc. Mass Spectrom., 2004, vol. 15, pp. 1287–1299. DOI: 10.1016/j.jasms.2004.06.003.
Patent 2158598 (RU). 2000. (in Russ.).
Gosudarstvennaya farmakopeya XIII izd. OFS 1.2.1.0005.15. [State Pharmacopoeia. Ed. XIII. Official Pharmacopeia Ar-ticle 1.2.1.0005.15]. (in Russ.).
De Souza R.F.V., De Giovani W.F. Spectrochimica Acta Part A., 2005, vol. 61, pp. 1985–1990. DOI: 10.1016/j.saa.2004.07.029.
Cornard J.P., Merlin J.C. Journal of Inorganic Biochemistry, 2002, vol. 92, pp. 19–27.
Zheltoukhova Ye.P., Koval'chukova O.V., Zaytsev B.Ye., Strashnova S.B. Novyye dostizheniya v khimii i khimicheskoy tekhnologii rastitel'nogo syr'ya: materialy IV Vserossiyskoy konferentsii. [New advances in chemistry and chemical tech-nology of plant materials: materials of the IV All-Russian Conference]. Barnaul, 2009, vol. 2, pp. 217–218. (in Russ.).
Torreggiani A., Tamba M., Trinchero A., Bonora S. Journal of Molecular structure, 2005, vol. 744–747, pp. 759–766. DOI: 10.1016/j.molstruc.2004.11.081.
Cornard J.P., Boudet A.C., Merlin J.C. Spectrochimica Acta Part A, 2001, vol. 57, issue 3, pp. 591–602.
Chervyakovskiy Ye.M., Kurchenko V.P., Kostyuk V.A. Trudy Belorusskogo gosudarstvennogo universiteta, 2009, vol. 4, part 1, pp. 9–26. (in Russ.).
Heim K.E., Tagliaferro A.R., Bobilya D.J. Journal of Nutritional Biochemistry, 2002, vol. 13, pp. 572–584.
Panhwar Q.K., Memon Sh. Inorganica Chimica Acta, 2013, vol. 407, pp. 252–260. DOI: 10.1016/j.ica.2013.08.001.
Shchekatikhina A.S., Kurchenko V.P. Trudy Belorusskogo gosudarstvennogo universiteta, 2011, vol. 6, part 1, pp. 76–85. (in Russ.).
Zenkevich I.G., Yeshchenko YU.A., Makarov V.G., Kolesnik YU.A., Shmatkov D.A., Tikhonov V.P., Tashlitskiy V.M. Aktual'nyye problemy sozdaniya novykh lekarstvennykh preparatov prirodnogo proiskhozhdeniya. Fitofarm-2006: materialy KH mezhdunarodnogo s"yezda. [Actual problems of creating new drugs of natural origin. Phytopharm 2006: materials of the 10th international congress]. St. Petersburg, 2006, pp. 93–109. (in Russ.).
Vyaznikova M.YU., Nikolayeva S.S., Smirnova L.P., Bykov V.A. Khimiko-farmatsevticheskiy zhurnal, 1997, vol. 31, no. 2, pp. 39–41. (in Russ.).
Vyaznikova M.YU., Nikolayeva S.S., Bykov V.A., Yakovleva L.V., Rulenko I.A., Tyukavkina N.A., Kolesnik YU.A. Khimiko-farmatsevticheskiy zhurnal, 1997, vol. 31, no. 2, pp. 42–45. (in Russ.).
Selifonova Ye.I., Chernova R.K., Koblova O.Ye. Izvestiya Saratovskogo universiteta. Novaya seriya. Seriya: Khimiya. Biologiya. Ekologiya, 2008, vol. 8, no. 2, pp. 23–28. (in Russ.).
Tarasevich B.N. IK spektry osnovnykh klassov organicheskikh soyedineniy. Spravochnyye materialy. [IR spectra of the main classes of organic compounds. Reference materials]. Moscow, 2012, 54 p. (in Russ.).
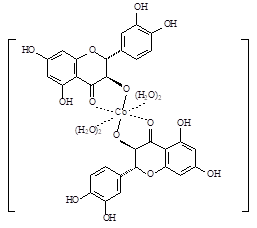

This work is licensed under a Creative Commons Attribution 4.0 International License.
The authors, which are published in this journal, agree to the following conditions:
1. Authors retain the copyright to the work and transfer to the journal the right of the first publication along with the work, at the same time licensing it under the terms of the Creative Commons Attribution License, which allows others to distribute this work with the obligatory indication of the authorship of this work and a link to the original publication in this journal .
2. The authors retain the right to enter into separate, additional contractual agreements for the non-exclusive distribution of the version of the work published by this journal (for example, to place it in the university depository or to publish it in a book), with reference to the original publication in this journal.
3. Authors are allowed to post their work on the Internet (for example, in a university repository or on their personal website) before and during the review process of this journal, as this may lead to a productive discussion, as well as more links to this published work.











