SYNTHESIS OF TRITERPENE POLYMER CONSTRUCTIONS
UDC 547.597:541.64:615.277.3
Abstract
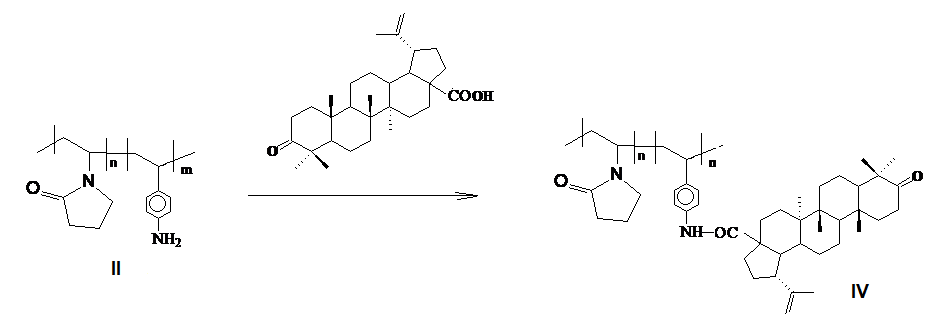
The pentacyclic triterpenoids betulin and betulonic acid are promising sources of new biologically active compounds. Grafting them onto polymer matrices leads to the formation of effective dosage forms compared to the original drug. On the basis of betulin and betulonic acid, the synthesis of polymer structures with a fragment of triterpene in the side chain was carried out. New triterpene-containing polymer ensembles were obtained by the method of polymer-analogous transformations of copolymers of N-vinylpyrrolidone with N-(n-carboxy) phenylmaleimide and p-aminostyrene. By crosslinking the carboxyl groups of the copolymer of N-vinylpyrrolidone with N-(n-carboxy) phenylmaleimide with betulin hydroxyl groups and the reaction of the amino groups of the copolymer of N-vinyl pyrrolidone with p-aminopyrol with carboxyl groups of betulonic acid, polymer structures with polycyclic triterpene fragments are obtained. The resulting polymer ensembles have higher activity against melanoma compared to the original copolymers. A polymer based on a copolymer of N-vinylpyrrolidone with N-(n-carboxy) phenylmaleimide and betulin at a concentration of 40.48 μM inhibits 50% of MS cells, while 50% of MS cells die under the influence of 68.29 μM betulin. New triterpene-containing polymer ensembles are promising for the development of new biologically active polymer bioconjugates.
Downloads
Metrics
References
Tolstikov G.A., Flekhter O.B., Shul'ts E.E., Baltina L.A., Tolstikov A.G. Khimiya v interesakh ustoychivogo razvitiya, 2005, vol. 13, pp. 1–30. (in Russ.).
Yogeeswari P., Sriram D. Curr. Med. Chem., 2005, vol. 12, pp. 657–666.
Tolstikova Т.G., Sorokina I.V., Tolstikov G.А., Tolstikov А.G., Flekhter О.B. Rus. J. Bioorg. Chem., 2006, vol. 32, no. 3, pp. 261–276. DOI: 10.1134/S1068162006030083.
Alakurtti S., Mäkelä T., Koskimies S., Yli-Kauhaluoma J. Europ. J. Pharm. Sciences, 2006, vol. 29, no. 1, pp. 1–13. DOI: 10.1016/j.ejps.2006.04.006.
Krasutsky P.A. Nat. prod. rep., 2006, vol. 23, no. 6, pp. 919–942.
Tolstikova Т.G., Sorokina I.V., Tolstikov G.А., Tolstikov А.G., Flekhter О.B. Rus. J. Bioorg. Chem., 2006, vol. 32, no. 1, pp. 37–49. DOI: 10.1134/S1068162006010031.
Yu D., Wild C.T., Martin D.E., Morris-Natschke S.L., Chen C.H., Allaway G.P., Lee K.H. Expert Opin. Investig. Drugs, 2005, vol. 14, pp. 681–693. DOI: 10.1517/13543784.14.6.681
Lee K.-H. J. Nat. Prod., 2010, vol. 73, pp. 500–516. DOI: 10.1021/np900821e.
Qian K., Morris-Natschke S.L., Lee K.H. Med. Res. Rev., 2009, vol. 29, pp. 369–393. DOI: 10.1002/med.20138.
Pichette A., Liu H., Roy C., Tanguay S., Simard F., Lavoie S. Synth. Commun., 2004, vol. 34, no. 21, pp. 3925–3937. DOI: 10.1081/SCC-200034788.
Era V., Jaaskelamen P., Ukkonen К. J. Amer. Oil Chem. Soc., 1981, vol. 58, no. 1, pp. 20–23.
Era V., Mustonen Т., Jaaskelainen Р. Mackromol. Chem. Rapid Commun., 1981, vol. 2, pp. 283–286. DOI: 10.1002/marc.1981.030020407.
Patent 1671666 (USSR). 1991. (in Russ.).
Gorbunova M.N., Krainova G.F., Tolmacheva I.A., Grishko V.V. Russ. J. Appl. Chem., 2012, vol. 85, no. 7, pp. 1137–1141. DOI: 10.1134/S1070427212070269.
Krainova G.F., Tolmacheva I.A., El’tsov O.S., Gorbunova M.N., Grishko V.V. Chem. Nat. Compd., 2016, vol. 52, no. 2, pp. 256–261. DOI: 10.1007/s10600-016-1608-5.
Gorbunova M.N., Krainova G.F., Kisel’kov D.M., Nebogatikov V.O. Russ. J. Appl. Chem., 2016, vol. 89, no. 3, pp. 437–444. DOI: 10.1134/S1070427216030149.
Krainova G.F., Tolmacheva I.A., Gorbunova M.N., Grishko V.V. Chem. Nat. Comp., 2013, vol. 49, no. 2, pp. 281–285. DOI: 10.1007/s10600-013-0582-4.
Gorbunova M.N., Krainova G.F., Tolmacheva I. A., Grishko V.V. Russ. J. Bioorg. Chem., 2015, vol. 41, no. 7, pp. 732–738. DOI: 10.1134/S1068162015070043.
Flekhter O.B., Nigmatullina L.R., Baltina L.A., Karachurina L.T., Galin F.Z., Zarudiy F.S., Tolstikov G.A., Bore-ko Ye.I., Pavlova N.I., Nikolayeva S.N., Savinova O.V. Khimiko-farmatsevticheskiy zhurnal, 2002, vol. 36, no. 9, pp. 26–28. (in Russ.).
Flekhter O.B., Ashavina O.Yu., Boreko Ye.I., Karachurina L.T., Pavlova N.I., Kabal'nova N.N., Savinova O.V., Galin F.Z., Nikolayeva S.N., Zarudiy F.S., Baltina L.A., Tolstikov G.A. Khimiko-farmatsevticheskiy zhurnal, 2002, vol. 36, no. 6, pp. 21–24. (in Russ.).
Fizer L., Fizer M. Reagenty dlya organicheskogo sinteza. [Reagents for organic synthesis]. Moscow, 1971, vol. 4, p. 49. (in Russ.).
Scudiero D.A., Shoemaker R.H., Paull K.D., Monks A., Tierney S., Notziger T.H., Currens M.T., Seniff D., Boyd M.K. Cancer Res., 1988, vol. 48, pp. 4827–4833.
Copyright (c) 2020 Khimiya Rastitel'nogo Syr'ya (Chemistry of plant raw material)

This work is licensed under a Creative Commons Attribution 4.0 International License.

This work is licensed under a Creative Commons Attribution 4.0 International License.
The authors, which are published in this journal, agree to the following conditions:
1. Authors retain the copyright to the work and transfer to the journal the right of the first publication along with the work, at the same time licensing it under the terms of the Creative Commons Attribution License, which allows others to distribute this work with the obligatory indication of the authorship of this work and a link to the original publication in this journal .
2. The authors retain the right to enter into separate, additional contractual agreements for the non-exclusive distribution of the version of the work published by this journal (for example, to place it in the university depository or to publish it in a book), with reference to the original publication in this journal.
3. Authors are allowed to post their work on the Internet (for example, in a university repository or on their personal website) before and during the review process of this journal, as this may lead to a productive discussion, as well as more links to this published work.











