SULFATION OF ABIES ETHANOL LIGNIN WITH COMPLEXES OF SULFUR TRIOXIDE WITH 1,4-DIOXANE AND PYRIDINE
UDC 547.993:543.421/424:543.429
Abstract
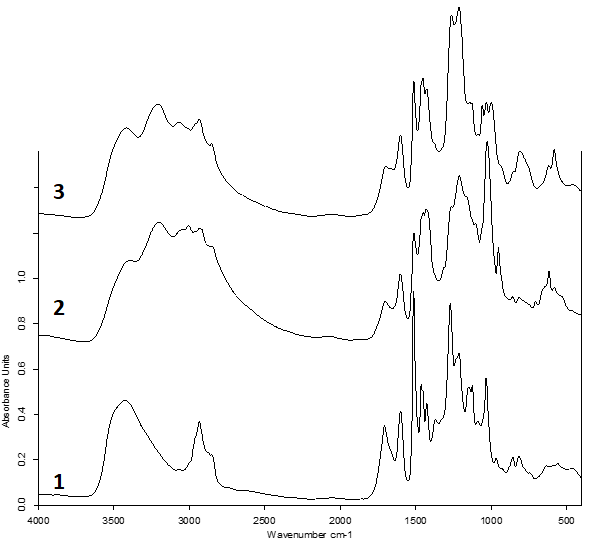
In this work, we optimized the process of sulfating abies ethanol lignin with complexes of sulfuric anhydride with pyridine and 1,4-dioxane. Experimentally found are the conditions for the implementation of the process of sulfation of abies ethanol lignin by complexes of sulfur trioxide with 1,4-dioxane and pyridine, providing a high sulfur content (12.0–12.6%). It was shown that a high sulfur content of 12.0–13.5% (mass.) in the obtained ethanol lignin sulfate is achieved when the ratio of the amount of chlorosulfonic acid to the amount of abies ethanol lignin is 20.22 : 1 mmol : g and the duration of the sulfation process is 60–120 min and independent of the nature of the sulfating complex. The structure and composition of water-soluble sulfated abies ethanol lignin are confirmed by FTIR spectroscopy, gel permeation chromatography and elemental analysis. In the FTIR spectra of sulfated abies ethanol lignin, in comparison with the FTIR spectra of the initial abies ethanol lignin, there are absorption bands in the region of 1270–1260, 1220–1212, 861–803 cm-1, corresponding to vibrations of sulfate groups. Compared to the initial lignin, sulfated abies ethanol lignin has a low degree of polydispersity. In particular, there was an increase in Mw c ~1.5 kDa to ~3.4 kDa in lignin sulfated for 30 min and a decrease in polydispersity from 2.59 to 1.22 compared to the initial abies ethanol lignin. With an increase in the sulfation time, the profile of the molecular mass distribution curve shifts to a high molecular weight region, with a simultaneous increase in polydispersity to 1.5 and Mw increases to ~4.3 kDa.
Downloads
Metrics
References
Liu Q., Luo L., Zheng L. International Journal of Molecular Sciences, 2018, vol. 19, no. 2, pp. 335–341. DOI: 10.3390/ijms19020335.
Lourenço A., Pereira H. Lignin – trends and applications, IntechOpen, 2018, pp. 65–98. DOI: 10.5772/intechopen.71208.
Brauns F., Hibbert H. Canadian Journal of Research, 1935, vol. 13b(1), pp. 28–34. DOI: 10.1139/cjr35b-003.
Tribot A., Amer G., Alio M.A., Baynast H., Delattre C., Pons A., Mathias J.-D., Callois J.-M., Vial C., Michaud P., Dussap C.-G. European Polymer Journal, 2019, vol. 112, pp. 228–240. DOI: 10.1016/j.eurpolymj.2019.01.007.
Danish M., Ahmad T. Renewable and Sustainable Energy Reviews, 2018, vol. 87, pp. 1–21. DOI: 10.1016/j.rser.2018.02.003.
Spiridon I. Cellulose Chemistry and Technology, 2018, vol. 52, no. 7–8, pp.543–550.
Witzler M., Alzagameem A., Bergs M., Khaldi-Hansen B.E., Klein S.E., Hielscher D., Schulze M. Molecules, 2018, vol. 23, no. 8, p. 1885. DOI: 10.3390/molecules23081885.
Vinardell M.P., Mitjans M. International Journal of Molecular Sciences, 2017, vol. 18, no. 6, p. 1219. DOI: 10.3390/ijms18061219.
Andrei G., Lisco A., Vanpouille C., Introini A., Balestra E., van den Oord J., Cihlar T., Perno C.F., Snoeck R., Margo-lis L., Balzarini J. Cell Host Microbe, 2011, vol. 10, pp. 379–389. DOI: 10.1016/j.chom.2011.08.015.
Raghuraman A., Tiwari V., Zhao Q., Shukla D., Debnath A.K., Desai U.R. Biomacromolecules. 2007, vol. 8, pp. 1759–1763. DOI: 10.1021/bm0701651.
Saluja B., Thakkar J.N., Li H., Desai U.R., Sakagami M. Pulmonary Pharmacology and Therapeutics, 2013, vol. 26, pp. 296–304. DOI: 10.1016/j.pupt.2012.12.009.
Barapatre A., Aadil K.R., Tiwary B.N., Jha H. International Journal of Biological Macromolecules, 2015, vol. 75, pp. 81–89. DOI: 10.1016/j.ijbiomac.2015.01.012.
Hasegawa Y., Kadota Y., Hasegawa C., Kawiminami S. Journal of Nutritional Science and Vitaminology. 2015, vol. 61, pp. 449–454. DOI: 10.3177/jnsv.61.449.
Pan X., Kadla J.F., Ehara K., Gilkes N., Saddler J.N. Journal of Agricultural and Food Chemistry, 2006, vol. 54, pp. 5806–5813. DOI: 10.1021/jf0605392.
Qazi S.S., Li D., Briens C., Berruti F., Abou-Zaid M.M. Molecules, 2017, vol. 22, E372. DOI: 10.3390/molecules22030372.
Sun S.N., Cao X.F., Xu F., Sun R.C., Jones G.L. Journal of Agricultural and Food Chemistry. 2014, vol. 62, pp. 5939–5947. DOI: 10.1021/ jf5023093.
Barapatre A., Meena A.S., Mekala S., Das A., Jha H. International Journal of Biological Macromolecules, 2016, vol. 86, pp. 443–453. DOI: 10.1016/j. ijbiomac.2016.01.109.
Wang Q., Mu H., Zhang L., Dong D., Zhang W., Duan J. International Journal of Biological Macromolecules, 2015, vol. 74, pp. 507–514. DOI: 10.1016/j. ijbiomac.2014.12.044.
Frangville C., Rutkevicius M., Richter A.P., Velev O.D., Stoyanov S.D., Paunov V.N. Chemphyschem, 2012, vol. 13, pp. 4235–4243. DOI: 10.1002/cphc.201200537.
Richter A., Brown J.S., Bharti B., Wang A., Gangwal S., Houck K., Cohen Hubal E.A., Paunov V.N., Stoyanov S.D., Velev O.D. Nature Nanotechnology, 2015, vol. 10, pp. 817–823. DOI: 10.1038/nnano.2015.141.
Prinsen P., Narani A., Hartog A.F., Wever R., Rothenberg G. ChemSusChem. 2017, vol. 10, no. 10, pр. 2267–2273. DOI: 10.1002/cssc.201700376.
Liang A., Thakkar J.N., Hindle M., Desai U.R. Journal of Chromatography B, 2012, vol. 908, pp. 45–51. DOI: 10.1016/j.jchromb.2012.09.036.
Henry B.L., Thakkar J.N., Liang A., Desai U.R. Biochemical and Biophysical Research Communications, 2012, vol. 417, no. 1, pp. 382–386. DOI: 10.1016/j.bbrc.2011.11.122.
Henry B.L., Desai U.R. Thrombosis Research, 2014, vol. 134, no. 5, pр. 1123–1129. DOI: 10.1016/j.thromres.2014.08.024.
Abdel-Aziz M.H., Mosier P.D., Desai U.R. Biochemical and Biophysical Research Communications, 2011, vol. 413, no. 2, pp. 348–352. DOI: 10.1016/j.bbrc.2011.08.102.
Raghuraman A., Tiwari V., Zhao Q., Shukla D., Debnath A.K., Desai U.R.. Biomacromolecules, 2007, vol. 8, pp. 1759–1763. DOI: 10.1021/bm0701651.
Thakkar J.N. Discovery of lignin sulfate as a potent ingibitor of HSV entry cells. Theses and Dissertations Graduate School, Virginia Commonwealth University, 2006, 132 p.
Raghuraman A., Tiwari V., Thakkar J.N., Gunnarsson G.T., Shukla D., Hindle M., Desai U.R. Biomacromolecules, 2005, vol. 6, pp. 2822–2832. DOI: 10.1021/bm0503064.
Dzhil'bert E.Ye. Sul'firovaniye organicheskikh soyedineniy. [Dzhil'bert E.E. Sulfonation of organic compounds]. Mos-cow, 1969, 416 p. (in Russ.).
Patent 2641758 (RU). 2018. (in Russ.).
Kuznetsov B.N., Vasilyeva N.Yu., Kazachenko A.S., Skvortsova G.P., Levdansky V.A., Lutoshkin M.A. Journal of Siberian Federal University. Chemistry, 2018, vol. 1, no. 11, pр. 22–130. DOI: 10.17516/1998-2836-0063.
Quesada-Medina J., López-Cremades F.J., Olivares-Carrillo P. Bioresource Technology, 2010, vol. 101, pр. 8252–8260. DOI: 10.1016/j.biortech.2010.06.011.
Cheronis N.D., Ma T.S. Mikro- i polumikrometody organicheskogo funktsional'nogo analiza. [Micro- and semi-micromethods of organic functional analysis]. Moscow, 1973, 576 p. (in Russ.).
Sudakova I.G., Garyntseva N.V., Yatsenkova O.V., Kuznetsov B.N. Journal of Siberian Federal University. Chemis-try, 2013, vol. 6, pp. 76–84.
Calvo-Flores F.G., Dobado J.A., Isac-García J., Martín-Martínez F.J. Lignin and Lignans as Renewable Raw Materi-als: Chemistry, Technology and Applications, John Wiley & Sons. Chichestes, 2015, 506 p.
Gosudarstvennaya farmakopeya Rossiyskoy Federatsii. 14 izd. [State Pharmacopoeia of the Russian Federation. 14th ed.]. Moscow, 2008, vol. 1, 704 p. (in Russ.).
Zakis G.F. Funktsional'nyy analiz ligninov i ikh proizvodnykh. [Functional analysis of lignins and their derivatives]. Ri-ga, 1987, 230 p. (in Russ.).
Khergert G.L. IK-spektry lignina. [IR spectra of lignin]. Moscow, 1975, 632 p. (in Russ.).
Bellamy L.J. Advances in Infrared Group Frequencies, London, 1968, 328 p.
Roeges N.P.G. A guide to the complete interpretation of infrared spectra of organic structures, John Wiley & Sons, 1995, 340 p.
Copyright (c) 2020 chemistry of plant raw material

This work is licensed under a Creative Commons Attribution 4.0 International License.

This work is licensed under a Creative Commons Attribution 4.0 International License.
The authors, which are published in this journal, agree to the following conditions:
1. Authors retain the copyright to the work and transfer to the journal the right of the first publication along with the work, at the same time licensing it under the terms of the Creative Commons Attribution License, which allows others to distribute this work with the obligatory indication of the authorship of this work and a link to the original publication in this journal .
2. The authors retain the right to enter into separate, additional contractual agreements for the non-exclusive distribution of the version of the work published by this journal (for example, to place it in the university depository or to publish it in a book), with reference to the original publication in this journal.
3. Authors are allowed to post their work on the Internet (for example, in a university repository or on their personal website) before and during the review process of this journal, as this may lead to a productive discussion, as well as more links to this published work.











