QUANTITATIVE DETERMINATION OF ARTEANNUIN B – A SESQUITERPENE LACTONE FROM ARTEMISIA ANNUA
UDC 547-314+54.056+615.07+615.32
Abstract
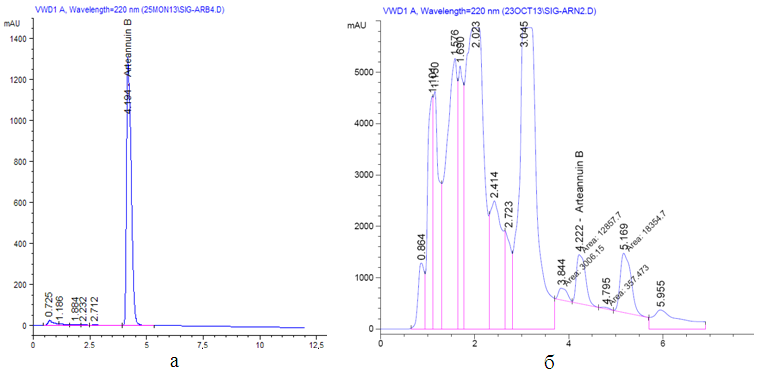
The HPLC (High performance liquid chromatography) method for the determination of arteannuin B in the herb Artemisia annua has been developed. The method is suitable for its validation characteristics, such as specificity parameters (convergence of the retention times of artianuin B in the standard and sample), suitability of the chromatographic system (separation efficiency), linearity (R2 = 0.99929 at y = 9.38x + 245.98), range of application (from 50 to 150%), accuracy in terms of repeatability (RSD = 3.45%) and correctness (Z = 100.36 ± 0.56%), determined experimentally, are within the recommended values. As a marker compound, it was proposed to use a standard sample of arteannuin B. For this, a standard sample of arteannuin B was obtained with a purity of at least 99%, which is confirmed IR, 1HNMR, 13CNMR spectral data. Organoleptic and physicochemical parameters were determined. It has been established that the presence of impurities in an amount of not more than 1% is allowed in a standard sample of arteannuin B. It was established that the content of arteannuin B in the raw material should be at least 0.2%. The development and validation HPLC-UV method for the determination of arteannuin B have to used to standardization the herb of Artemisia annua.
Downloads
Metrics
References
Konovalov D.A., Shevchuk O.M., Logvinenko L.A., Khamilonov A.A. Farmatsiya i farmakologiya, 2016, vol. 4, no. 5, pp. 4–35. DOI: 10.19163/2307-9266-2016-4-5-4-35. (in Russ.).
Makhatov B.K., Patsayev A.K., Konovalov D.M., Alikhanova Kh.B., Orynbasarova K.K., Sapakbay M.M., Rakhmanova G.S. Vestnik Kazakhstanskoy akademii yestestvennykh nauk, 2017, no. 3, pp. 86–90. (in Russ.).
Konovalov D.A., Tiraspol'skaya C.G., Alfimova G.V., Samoryadova A.B. Sovremennyye problemy nauki i obra-zovaniya, 2013, no. 4. URL: http://www.science-education.ru/ru/article/view?id=9735. (in Russ.).
Weathers P.J., Arsenault P.R., Covello P.S., McMickle A., Teoh K.H., Reed D.W. Phytochem Rev., 2011, vol. 10, pp. 173–183. DOI: 10.1007/s11101-010-9166-0.
Rastitel'nyye resursy SSSR. Tsvetkovyye rasteniya, ikh khimicheskiy sostav, ispol'zovaniye [Plant resources of the USSR. Flowering plants, their chemical composition, use], ed. P.D. Sokolov. St.-Petersburg, 1993, vol. 7, 361 p. (in Russ.).
Chaturvedi D., Goswami A., Saikia P.P., Barua N.C, Rao P.G. Chem. Soc. Rev., 2010, vol. 39(2), pp. 435–454. DOI: 10.1039/b816679j.
Meunier B., Robert A. Acc. Chem. Res., 2010, vol. 43(11), pp. 1444–1451. DOI: 10.1021/ar100070k.
Nakase I., Lai H., Singh N.P., Sasaki T. Int. J. Pharm., 2008, vol. 354(1), pp. 28–33. DOI: 10.1016/j.ijpharm.2007.09.003.
Chang Z. Sci. China Life Sci., 2016, vol. 59, no. 1, pp. 81–88. DOI: 10.1007/s11427-015-4988-z.
Klayman D.L. Science, 1985, vol. 228, no. 4703, pp. 1049–1055. DOI: 10.1126/science.3887571.
Islamova Zh.I., Mukhamatkhanova R.F., Dusmatova D.E., Sham'yanov I.D., Khushbaktova Z.A., Syrov V.N. Doklady Akademii Nauk RUz, 2017, no. 5, pp. 78–81. (in Russ.).
Islamova Zh.I., Sham'yanov I.D., Khushbaktova Z.A., Syrov V.N. Zhurnal teoreticheskoy i klinicheskoy meditsiny, 2019, no. 4, pp. 11–14. (in Russ.).
Zhang Y.Q., Ding W., Zhao Zhi-Mo, Wu J., FanY.-H. Agricultural Sciences in China, 2008. vol. 7, no. 5, pp. 577–584.
Kurkina A.V. Izvestiya Samarskogo tsentra Rossiyskoy akademii nauk, 2012, vol. 14, no. 1(9), pp. 2253–2256. (in Russ.).
Olsson M.E., Olofsson L.M., Lindahl A.-L., Lungren A., Brodelius M., Brodelius P.E. Phytochemistry, 2009, vol. 70, pp. 1123–1128. DOI: 10.1016/j.phytochem.2009.07.009.
Covello P.S., Teoh K.H., Polichuk D.R., Reed D.W., Nowak G. Phytochemistry, 2007, vol. 68, pp. 1864–1871. DOI: 10.1016/j.phytochem.2007.02.016.
Diawara H.Z., Gbaguidi F., Evrard B., Leclercq J.Q., Moudachirou M., Debrus B., Hubert P., Rozet E. Journal of Pharmaceutical and Biomedical Analysis, 2011, vol. 56, no. 1, pp. 7–15. DOI: 10.1016/j.jpba.2011.04.012.
Edwards G. Transactions of the Royal Society of Tropical Medicine and Hygiene, 1994, vol. 88, pp. 37–39. DOI: 10.1016/0035-9203(94)90470-7.
Widmer V., Handloser D., Reich E. J. Liq. Chrom. Relat. Tech., 2007, vol. 30, no. 15, pp. 2209–2219. DOI: 10.1080/10826070701451555.
Lapkin A.A., Walker A., Sullivan N., Khambay B., Mlambo B., Chemat S. Summary of Report on the Medicines for Malaria Ventures Supported Project, 2009. 7 p.
Soktoyeva T.E., Ryzhova G.L., Dychko K.A., Khasanov V.V., Zhigzhitzhapova S.V., Radnayeva L.D. Khimiya ras-titel'nogo syr'ya, 2011, no. 4, pp. 131–134. (in Russ.).
The International Pharmacopoeia. Ninth Edition, 2019, 3 p.
Copyright (c) 2021 chemistry of plant raw material

This work is licensed under a Creative Commons Attribution 4.0 International License.

This work is licensed under a Creative Commons Attribution 4.0 International License.
The authors, which are published in this journal, agree to the following conditions:
1. Authors retain the copyright to the work and transfer to the journal the right of the first publication along with the work, at the same time licensing it under the terms of the Creative Commons Attribution License, which allows others to distribute this work with the obligatory indication of the authorship of this work and a link to the original publication in this journal .
2. The authors retain the right to enter into separate, additional contractual agreements for the non-exclusive distribution of the version of the work published by this journal (for example, to place it in the university depository or to publish it in a book), with reference to the original publication in this journal.
3. Authors are allowed to post their work on the Internet (for example, in a university repository or on their personal website) before and during the review process of this journal, as this may lead to a productive discussion, as well as more links to this published work.











