ANALYSIS OF FLAVONES C-GLYCOSIDES AND AND STEPWISE HYDROLYSIS OF THEIR ACETATES IN THE LEAVES OF RUBUS CHAMAEMORUS L.
UDC 54.061:54.062:615.322
Abstract
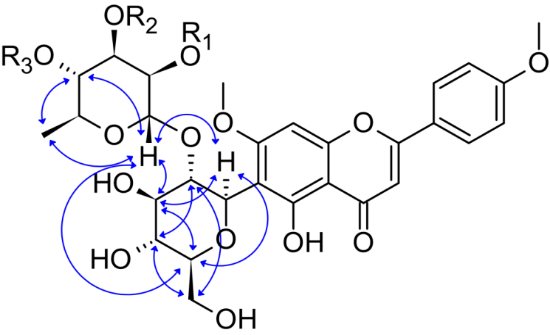
The C-glycoside embinin and its mono- and diacetate derivatives have immunotropic and cardiotonic activity, which makes the search for plants that contain them interesting. Embinin and its acetate derivatives were previously isolated only from some plants of the genus Iris, the habitat and growing conditions of which are very different from those of the genus Rubus. As a result of the study, the structure of seven C-glycosides, embinin derivatives, isolated from the leaves of Rubus chamaemorus L. (Rosaceae) was established. Using HR-ESI-MS, HPLC-MS, as well as one- and two-dimensional NMR spectroscopy, the structure of three substances isolated in individual form was established: embinin (1) and its diacetyl derivatives – 2''',3'''-diacetylembinin (5) and 3''',4'''-diacetylembinin (7). The method of stepwise hydrolysis of C-glycoside acetate residues proposed in this study, followed by HPLC analysis of the resulting hydrolysis products, made it possible to establish the structure of minor flavone C-glycosides contained in the leaves of Rubus chamaemorus L.: 2'''-acetylembinin (2), 3'''-acetylembinin (3), 4'''-acetylembinin (4) and 2''',4'''-diacetylembinin (6). All these compounds were found in the leaves of Rubus chamaemorus L. for the first time. The C-glycosides - embinin and its acetate derivatives are rare metabolites of higher plants, the presence of which is determined by the peculiarity of their physiology, and the biological activity determines the prospects for medical use.
Downloads
Metrics
References
Taylor K. Journal of Ecology, 1971, vol. 59, pp. 293–306.
Lohi K. Aquilo Ser. Botanica, 1974, vol. 13, p. 19.
Korpelainen H. Annals of Botany, 1994, vol. 74, pp. 627–632. DOI: 10.1006/anbo.1994.1164.
Barbara T. Biology Letters, 2003, vol. 40, no. 1, pp. 3–13.
McDougall G.J., Martinussen I., Junttila O., Verrall S., Stewart D. Journal of Agricultural and Food Chemistry, 2011, vol. 59, no. 20, pp. 10860–10868. DOI: 10.1021/jf202083b.
Kähkönen M.P., Hopia A.I., Heinonen M. Journal of Agricultural and Food Chemistry, 2001, vol. 49, no. 8, pp. 4076–4082. DOI: 10.1021/jf010152t.
Kähkönen M., Kylli P., Ollilainen V., Salminen J., Heinonen M. Journal of agricultural and food chemistry, 2012, vol. 60, no. 5, pp. 1167–1174. DOI: 10.1021/jf203431g.
Kaisu M., Afaf K., Törrönen A.R. Journal of Agricultural and Food Chemistry, 2004, vol. 52, no. 20, pp. 6178–6187. DOI: 10.1021/jf049450r.
Määttä-Riihinen K.R., Kamal-Eldin A., Törrönen A.R. Journal of Agricultural and Food Chemistry, 2004, vol. 52, no. 20, pp. 6178–6187. DOI: 10.1021/jf049450r.
Häkkinen S.H., Kärenlampi S.O., Heinonen I.M., Mykkänen H.M., Törrönen A.R. Journal of Agricultural and Food Chemistry, 1999, vol. 47, no. 6, pp. 2274–2279. DOI: 10.1021/jf9811065.
Ueyli A.K., Ponkratova A.O., Teslov L.S., Luzhanin V.G. Pul's, 2020, vol. 22, no. 7, pp. 50–59. (in Russ.).
Whaley A.K., Ebrahim W., El-Neketi M., Ancheeva E.U., Özkaya F.C., Pryakhina N.I., Sipkina N.U., Luzhanin V.G., Liu Z., Proksch P. Tetrahedron Letters, 2017, vol. 58, no. 22, pp. 2171–2173. DOI:10.1016/j.tetlet.2017.04.080.
Pryakhina N.I., Sheichenko V.I., Blinova K.F. Chemistry of Natural Compounds, 1984, vol. 20, no. 5, pp. 554–559.
Shen W.J., Qin M.J., Shu P., Zang C.F. Chinese Chemical Letters, 2008, vol. 19, no. 7, pp. 821–824. DOI: 10.1016/j.cclet.2008.04.031.
Kawase A., Yagishita K. Agricultural and Biological Chemistry, 1968, vol. 32, no. 4, pp. 537–538. DOI: 10.1080/00021369.1968.10859095.
Meng Y., Qin M., Qi B., Xie G. Phytochemistry Letters, 2017, vol. 22, pp. 33–38. DOI: 10.1016/j.phytol.2017.08.011.
Shen W.J., Qin M.J., Shu P., Zhang C.F. Chinese Chemical Letters, 2008, vol. 19, no. 7, pp. 821–824. DOI: 10.1016/j.cclet.2008.04.031.
Bychkova N.V., Kalashnikova A.A., Ueyli A.K., Luzhanin V.G., Kalinina N.M., Shustov Ye.B., Okovityy S.V. Pro-filakticheskaya i klinicheskaya meditsina, 2019, vol. 4, no. 73, pp. 77–82. (in Russ.).
Ivkin D.Yu., Luzhanin V.G., Karpov A.A., Minasyan S.M., Poleshchenko Ya.I., Mamedov A.E., Povydysh M.N., Poroykov V.V., Narkevich I.A. Razrabotka i registratsiya lekarstvennykh sredstv, 2018, vol. 3, no. 24, pp. 166–170. (in Russ.).
Rayyan S., Fossen T., Nateland H.S., Andersen Ø.M. Phytochemical Analysis, 2005, vol. 16, no. 5, pp. 334–341. DOI: 10.1002/pca.853.
Zhou G., Yan R., Wang X., Li S., Lin J., Liu J., Zhao Z. Phytochemistry Reviews, 2019, vol. 18, no. 2, pp. 443–461. DOI: 10.1007/s11101-019-09601-7.
Lewis K., Maxwell A., McLean S., Reynolds W., Enriquez R. Magnetic Resonance in Chemistry, 2000, vol. 38, pp. 771–774. DOI: 10.1002/1097-458X(200009)38:9<771::AID-MRC729>3.0.CO;2-4.
Copyright (c) 2021 chemistry of plant raw material

This work is licensed under a Creative Commons Attribution 4.0 International License.

This work is licensed under a Creative Commons Attribution 4.0 International License.
The authors, which are published in this journal, agree to the following conditions:
1. Authors retain the copyright to the work and transfer to the journal the right of the first publication along with the work, at the same time licensing it under the terms of the Creative Commons Attribution License, which allows others to distribute this work with the obligatory indication of the authorship of this work and a link to the original publication in this journal .
2. The authors retain the right to enter into separate, additional contractual agreements for the non-exclusive distribution of the version of the work published by this journal (for example, to place it in the university depository or to publish it in a book), with reference to the original publication in this journal.
3. Authors are allowed to post their work on the Internet (for example, in a university repository or on their personal website) before and during the review process of this journal, as this may lead to a productive discussion, as well as more links to this published work.











