INVESTIGATION OF SUPERCRITICAL CO2-EXTRACTS OF WILD LEDUM PALUSTRE L. (RHODODENDRON TO-MENTOSUM HARMAJA) AND IDENTIFICATION OF ITS METABOLITES BY TANDEM MASS SPECTROMETRY
UDC 615.322
Abstract
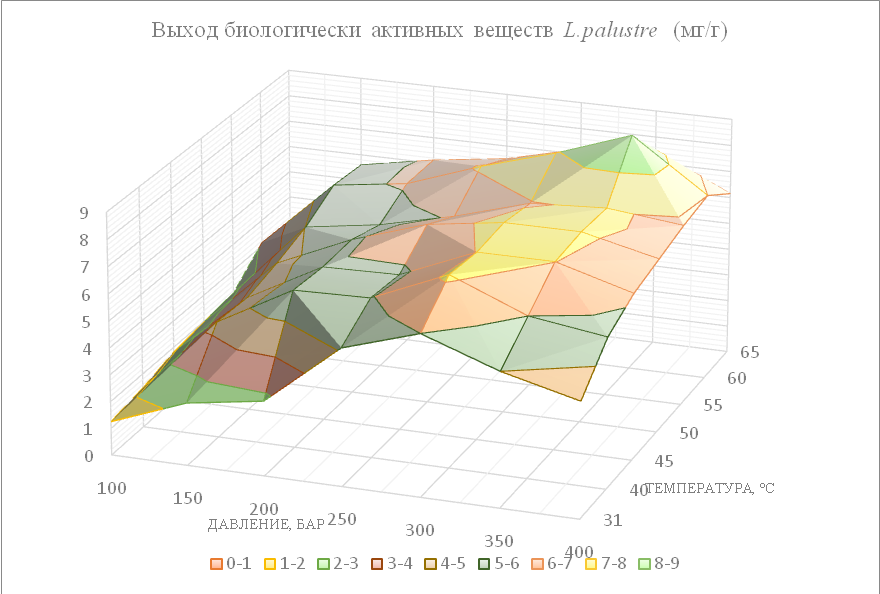
The purpose of this research is to investigate and identify polyphenolic complexes and other biologically active compounds by tandem mass spectrometry, presented in the leaves and stems of Ledum palustre L. Carbon dioxide, compressed to a supercritical state, was used for the most environmentally friendly extraction of polyphenolic complexes and other biologically active compounds of Ledum palustre L. The most effective extraction characteristics (pressure 350 bar; temperature 60 °С; extraction time 1-hour, co-solvent MeOH 3.5%) supercritical CO2-extraction of L. palustre were obtained empirically. To identify target analytes in supercritical extracts, High Performance Liquid Chromatography (HPLC) was used in combination with a BRUKER DALTONIKS ion trap. The results showed the presence of 61 biologically active compounds corresponding to the rhododendron species, of which 32 were identified for the first time in L. palustre. These are flavanols dihydrokaempferol, quercetin arabinoside, myricetin galactoside; flavones: diosmetin, nevadensin, cirsimaritin; flavanone naringenin; anthocyanins delphinidin, petunidin, cyanidin pentoside, delphinidin pentoside, peonidin 3-(6-O-acetyl) glucoside, peonidin-3-O-malonylglucoside, cyanidin-3-rutinoside, peonidin 3-O-glucoside; ellagic acid; lignan medioresinol; a type A procyanidin dimer; sterols fucosterol and avenasterol, etc.
Downloads
Metrics
References
Poyarkova A.I. Flora SSSR. [Flora of the USSR]. Moscow; Leningrad, 1952, vol. 18, pp. 31–60. (in Russ.).
Arsen'yev V.K. Po Ussuriyskomu krayu (Dersu Uzala). Puteshestviye v gornuyu oblast' Sikhote-Alin'. [In the Ussuri region (Dersu Uzala). Journey to the mountainous region of Sikhote-Alin]. Vladivostok, 1921, 280 p. (in Russ.).
Belousova N.I., Khan V.A., Tkachev A.V. Khimiya rastitel'nogo syr'ya, 1999, no. 3, pp. 5–38. (in Russ.).
Okhlopkova Zh.M., Chirikova N.K. Fundamental Research, 2012, no. 11, pp. 1334–1336. (in Russ.).
Bukreyeva T.V., Shavarda A.L., Matusevich O.V., Morozov M.A. Rastitel'nyye resursy, 2013, vol. 49, no. 2, pp. 395–403. (in Russ.).
Ganina M.M., Popova O.I. Khimiko-farmatsevticheskiy zhurnal, 2015, vol. 49, no. 4, pp. 33–35. (in Russ.).
Podmaskin V.V. Rossiya i ATR, 2011, no. 1(71), pp. 107–113. (in Russ.).
Izotov D.V. Efirnyye masla i vodomaslyanyye produkty vidov roda Ledum L., proizrastayushchikh na Dal'nem Vostoke: dis. … kand. biol. nauk. [Essential oils and water-oil products of species of the genus Ledum L. growing in the Far East: Cand. … cand. biol. Sciences]. Vladivostok, 2009, 235 p. (in Russ.).
Korotayeva M.S. Farmakognosticheskoye izucheniye chetyrekh vidov roda Ledum L.: dis. … kand. biol. nauk. [Phar-macognostic study of four species of the genus Ledum L.: dis. … cand. biol. Sciences]. Yaroslavl', 2006, 240 p. (in Russ.).
Plyashechnik M.A. Khimiya rastitel'nogo syr'ya, 2012, no. 2, pp. 139–144. (in Russ.).
Baldino L., Reverchon E. J. Supercrit. Fluids, 2018, vol. 134, pp. 269–273. DOI: 10.106/j.supflu.2017.11.034.
Popova A.S., Ivakhnov A.D., Skrebets T.E., Bogolitsyn K.G. Khimiya rastitel'nogo syr'ya, 2018, no. 1, pp. 61–66. DOI: 10.14258/jcprm.2018012994. (in Russ.).
Baananou S., Bagdonaite E., Marongiu B., Piras A., Porcedda S., Falconieri D., Boughattas N.A. Natural Product Re-search, 2015, vol. 29(11), pp. 999–1005. DOI: 10.1080/14786419.2014.965167.
Razgonova M.P., Zakharenko A.M., Grudev V., Ercisli S., Golokhvast K.S. Molecules, 2020, vol. 25(17), p. 3774. DOI: 10.3390/molecules25173774.
Gosudarstvennaya farmakopeya Rossiyskoy Federatsii. [State Pharmacopoeia of the Russian Federation]. Moscow, 2018, vol. 1–3. (in Russ.).
Abu-Reidah I. M., Ali-Shtayeh M.S., Jamous R.M., Arraes-Roman D., Segura-Carretero A. Food Chem., 2015, vol. 166, pp. 179–191. DOI: 10.1016/j.foodchem.2014.06.011.
Goufo P., Singh R.K., Cortez I. Antioxidants, 2020, vol. 9, p. 398. DOI: 10.3390/antiox9050398.
Hamed A.R., El-Hawary S.S., Ibrahim R.M., Abdelmohsen U.R., El-Halawany A.M. J. Chrom. Sci., 2021, vol. 59, pp. 618–626. DOI: 10.1093/chromsci/bmaa112.
Jaiswal R., Jayasinghe L., Kuhnert, N. J. Mass Spectrom., 2012, vol. 47, pp. 502–515. DOI: 10.1002/jms.2954.
Jin C., Strembiski W., Kulchytska Y., Micetich R.G., Daneshtalab M. DARU J. Pharm. Sci., 1999, vol. 7(4), pp. 5–8.
Li X., Tian T. Frontiers in Pharm., 2018, vol. 9, article 1067. DOI: 10.3389/fphar.2018.01067.
Llorent-Martinez E.J., Spinola V., Gouveia S., Castilho P. Industrial Crops and Products, 2015, vol. 69, pp. 80–90. DOI: 10.106/j.indcrop.2015.02.014.
Lommen A., Godejohann M., Venema D.P., Hollman P.C.H., Spraul M. Anal. Chem., 2000, vol. 72(8), pp. 1793–1797. DOI: 10.1021/ac9912303.
Pandey R., Kumar B. J. Liquid Chromatogr. & Related Technol., 2016, vol. 39(4), pp. 225–238. DOI: 10.1080/10826076.2016.1148048.
Rodriguez-Perez C., Gomez-Caravaca A.M., Guerra-Hernandez E., Cerretani L., Garcia-Villanova B., Verardo V. Food Res. Int., 2018, vol. 112, pp. 390–399. DOI: 10.1016/j.foodres.2018.06.060.
Ruiz A., Hermosin-Gutierrez I., Vergara C., von Baer D., Zapata M., Hitschfild A., Obando L., Mardones C. Food Res. Int., 2013, vol. 51(2), pp. 706–713. DOI: 10.1016/j.foodres.2013.01.043.
Sun J., Liu X., Yang T., Slovin J., Chen P. Food Chem., 2014, vol. 146, pp. 289–298. DOI: 10.1016/j.foodchem.2013.2013.08.089.
Sun L., Tao S., Zhang S. Molecules, 2019, vol. 24(1), p. 159. DOI: 10.3390/molecules24010159.
Vallverdu-Queralt A., Jauregui O., Medina-Remon A., Lamuela-Raventos R.M. Agricult. Food Chem., 2012, vol. 60(13), pp. 3373–3380. DOI: 10.1021/jf204702f.
Viera M.N., Winterhalter P., Jerz G. Phytochem. Anal., 2016, vol. 27, pp. 116–125. DOI: 10.1002/pca.2606.
Wang Z., Zhu W., Liu H., Wu G., Song M., Yang B., Yang D., Wang Q., Kuang H. Molecules, 2018, vol. 23(9), p. 2285. DOI: 10.3390/molecules23092285.
Wojakowska A., Perkowski J., Goral T., Stobiecki M. J. Mass. Spectrom., 2013, vol. 48, pp. 329–339. DOI: 10.1002/jms.3160.
Xiao J., Wang T., Li P., Liu R., Li Q., Bi K. J. Chromatogr. B, 2016, vol. 1028, pp. 33–41. DOI: 10.1016/j.jchromb.2016.06.005.
Xu L.L., Xu J.J., Zhong K.R., Shang Z.P., Wang F., Wang R.F., Liu B. Molecules, 2017, vol. 22(10), p. 1756. DOI: 10.3390/molecules22101756.
Yang S.T., Wu X., Rui W., Guo J., Feng Y.E. Acta Chromatogr., 2015, vol. 27(4), pp. 711–728. DOI: 10.1556/achrom.27.2015.4.9.
Zakharenko A.M., Razgonova M.P., Pikula K.S., Golokhvast K.S. Biochemistry Research International, 2021, article 9957490. DOI: 10.1155/2021/9957490.
Zhang Z., Jia P., Zhang X., Zhang Q., Yang H., Shi H., Zhang L. J. Ethnopharmacol., 2014, vol. 158, pp. 66–75. DOI: 10.1016/j.jep.2014.10.022.
Copyright (c) 2022 chemistry of plant raw material

This work is licensed under a Creative Commons Attribution 4.0 International License.

This work is licensed under a Creative Commons Attribution 4.0 International License.
The authors, which are published in this journal, agree to the following conditions:
1. Authors retain the copyright to the work and transfer to the journal the right of the first publication along with the work, at the same time licensing it under the terms of the Creative Commons Attribution License, which allows others to distribute this work with the obligatory indication of the authorship of this work and a link to the original publication in this journal .
2. The authors retain the right to enter into separate, additional contractual agreements for the non-exclusive distribution of the version of the work published by this journal (for example, to place it in the university depository or to publish it in a book), with reference to the original publication in this journal.
3. Authors are allowed to post their work on the Internet (for example, in a university repository or on their personal website) before and during the review process of this journal, as this may lead to a productive discussion, as well as more links to this published work.











