DYNAMICS OF ACCUMULATIONS IN LEAVES FLAVONOIDS AMARANTHUS RETROFLEXUS, AGASTACHE RUGOSA AND THLASPI ARVENSE GATHERED IN THE CENTRAL YAKUTIA
Abstract
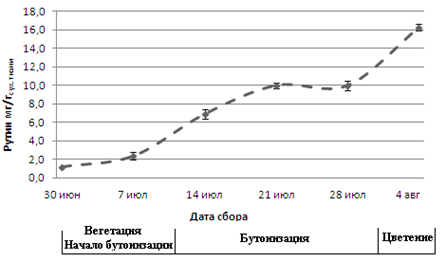
We investigated changes in the content of flavonoids in the leaves of Amaranthus retroflexus, Agastache rugosa and Thlaspi arvense, gathered in the Central Yakutia in different phenological phases. It is established that in leaves Amaranthus retroflexus, grown in the Central Yakutia contains rutin, in leaves Agastache rugosa – luteolin-7-O-glucoside, apigenin-7-O-glucoside, luteolin and apigenin, and leaves Thlaspi arvense – luteolin-7-O-glycoside. It is shown that the highest content of rutin in leaves Amaranthus retroflexus were in the flowering stage. It was revealed that the maximum concentration of luteolin-7-O-glucoside, luteolin and apigenin in leaves Agastache rugosa observed in the period of budding and flowering, and apigenin-7-O-glucoside – in the flowering stage. The content of luteolin-7-O-glucoside in Thlaspi arvense leaves was highest during budding and flowering. Thus, to obtain plant material with a maximum content of flavonoids in Amaranthus retroflexus, Agastache rugosa, Thlaspi arvense grown in Central Yakutia collecting should be carried out in the flowering stage.Downloads
Metrics
References
Andersen O.M., Markham K.R. Flavonoids: chemistry, biochemistry and applications. CRC Press, 2005. 1256 p.
Тараховский Ю.С., Ким Ю.А., Абдрасилов Б.С., Музафаров Е.Н. Флавоноиды: биохимия, биофизика, медицина. Пущино, 2013. 310 c.
Минаева В.Г. Флавоноиды в онтогенезе растений и их практическое использование. Новосибирск, 1978. 252 с.
Кершенгольц Б.М. Неспецифические биохимические механизмы адаптации организмов к экстремальным условиям среды // Наука и образование. 1996. Т. 3. С. 130–138.
Кершенгольц Б.М. Структурное разнообразие биологически активных веществ – биохимическая основа толерантности организмов в стрессовых условиях среды // Терпимость: идеи и традиции : материалы Международной научной конференции. Якутск, 1995. С. 179–184.
Шеин А.А., Прокопьев И.А., Филиппова Г.В., Журавская А.Н. Влияние техногенного загрязнения на содержание фотосинтетических пигментов и флавоноидов Matricaria Chamomila (Asteraceae) // Растительные ресурсы. 2014. №2. С. 235–241.
Лакин Г.Ф. Биометрия. М., 1980. 456 с.
Ломбоева С.С., Танхаева Л.М., Олейников Д.Н. Динамика накопления флавоноидов в надземной части ортилии однобокой (Orthilia Secunda (L.) House) // Химия растительного сырья. 2008. №3. С. 83–88.
Костикова В.А., Высочина Г.И., Петрук А.А. Особенности накопления флавоноидов в органах надземной части Rheum compactum L. // Химия растительного сырья. 2015. №4. С. 147–150.
Çιrak C., Radušienė J., Janulis V., Ivanauskas L. Secondary metabolites in Hypericum perfoliatum: variation among plant parts and phenological stages // Botanica Helvetica. 2007. Vol. 117. N1. Pp. 29–36.
Kazlauskas S., Bagdonaite E. Quantitative analysis of active substances in St. John's wort (Hypericum perforatum L.) by the high performance liquid chromatography method // Medicina. 2003. Vol. 40. N10. Pp. 975–981.
Kalinova J., Dadakova E. Rutin and total quercetin content in amaranth (Amaranthus spp.) // Plant foods for human nutrition. 2009. Vol. 64. N1. Pp. 68–74.
Vogelmann J.E. Flavonoids of Agastache section Agastache // Biochemical systematics and ecology. 1984. Vol. 12. N4. Pp. 363–366.
Llugany M., Tolrà R., Martín S.R., Poschenrieder C., Barceló J. Cadmium induced changes in glutathione and phenolics of Thlaspi and Noccaea species differing in Cd accumulation // Journal of Plant Nutrition and Soil Science. 2013. Vol. 176. N6. Pp. 851–858.
Formica J.V., Regelson W. Review of the biology of quercetin and related bioflavonoids // Food and chemical toxi-cology. 1995. Vol. 33. N12. Pp. 1061–1080.
Минаева В.Г., Горбалева Г.Н. О влиянии флавоноидов на прорастание пыльцы и рост пыльцевых трубок // Полезные растения природной флоры Сибири. Новосибирск, 1967. С. 231–237.
Harborne J.B., Williams C.A. Advances in flavonoid research since 1992 // Phytochemistry. 2000. Vol. 55. N6. Pp. 481–504.
Dai G.H., Nicole M., Andary C., Martinez C., Bresson E., Boher B., Daniel J.F., Geiger J.P. Flavonoids accumulate in cell walls, middle lamellae and callose-rich papillae during an incompatible interaction between Xanthomonas campestris pv. malvacearum and cotton // Physiological and Molecular Plant Pathology. 1996. Vol. 49. N5. Pp. 285–306.
Mierziak J., Kostyn K., Kulma A. Flavonoids as important molecules of plant interactions with the environment // Molecules. 2014. Vol. 19, N10. Pp. 16240–16265.

This work is licensed under a Creative Commons Attribution 4.0 International License.
The authors, which are published in this journal, agree to the following conditions:
1. Authors retain the copyright to the work and transfer to the journal the right of the first publication along with the work, at the same time licensing it under the terms of the Creative Commons Attribution License, which allows others to distribute this work with the obligatory indication of the authorship of this work and a link to the original publication in this journal .
2. The authors retain the right to enter into separate, additional contractual agreements for the non-exclusive distribution of the version of the work published by this journal (for example, to place it in the university depository or to publish it in a book), with reference to the original publication in this journal.
3. Authors are allowed to post their work on the Internet (for example, in a university repository or on their personal website) before and during the review process of this journal, as this may lead to a productive discussion, as well as more links to this published work.











