ANTIOXIDANT ACTIVITY OF TRITERPENE GLYCOSIDE (CORTUSOSIDE A) FROM CORTUSA MATTHIOLI L. PLANT
UDC 547.918:541.69
Abstract
The antioxidant activity of triterpene glycoside, first isolated from the aboveground part of the plant Cortusa matthioli L. and identified as β-D-xylopyranosyl-(1→2)-β-D-glucopyranosyl-(1→4)-[β-D-glucopyranosyl-(1→2)]-α-L-arabinopyranoside-(1→3)-13β,28-epoxyolean-30-al-3β,16α-diol (cortusoside A), is studied. Tests for the ability of cortusoside A to bind 2,2-diphenyl-1-picrylhydrazyl (DPPH) radicals did not reveal any activity of this compound. However, in experiments to study the ability to chelate Fe2+ ions, its sufficiently high iron chelating activity was found, which was only 2.24 times lower compared to the powerful Fe2+ chelator dipyridyl. The EC50 values for cortusoside A and dipyridyl were 0.417±0.057 and 0.186±0.018 mM, respectively. Literature analysis has shown that the structural analogue of cortusoside A, saxifragifolin B, has a much weaker iron chelating ability (13,4 times) compared to the standard Fe2+ EDTA-Na2 ion chelator, as well as a weak ability to bind free radicals of DPPH compared to the reference antioxidants – catechin and ascorbic acid (50 and 32 times, respectively). Despite the structural identity of the molecules cortusoside A and saxifragifolin B, low radiculopathy activity cortusosoide A may be due to differences in the structure of these substances (optical or geometric isomerism), as well as different methods were used in its definition.
Downloads
Metrics
References
Francis G., Keren Z., Makkar H.P.S., Becker K. British Journal of Nutrition, 2002, vol. 88, pp. 587–605.
Lupanova I.A., Mineyeva M.F., Kolkhir V.K., Martynov A.M. Sibirskiy meditsinskiy zhurnal, 2011, no. 6, pp. 244–246. (in Russ.).
Vasil'yeva I.S., Paseshnichenko V.A. Prikladnaya biokhimiya i mikrobiologiya, 1999, vol. 35, no. 5, pp. 521–535. (in Russ.).
Rastitel'nyye resursy SSSR: Tsvetkovyye rasteniya, ikh khimicheskiy sostav, ispol'zovaniye; Semeystva Paeoniaceae – Thymelaeaceae. [Plant resources of the USSR: Flowering plants, their chemical composition, use; Family Paeoniaceae – Thymelaeaceae]. Leningrad, 1986, vol. 2, 336 p. (in Russ.).
Asilbekova D.T., Nuriddinov Kh.R. Khimiya rastitel'nogo syr'ya, 2011, no. 4, pp. 219–222. (in Russ.).
Beshley I.V., Ufimtsev K.G., Volodin V.V., Shirshova T.I. Rastitel'nyye resursy, 2018, vol. 54, no. 4, pp. 532–541. DOI: 10.1134/S0033994618040040 (in Russ.).
Beshley I.V., Shirshova T.I., Volodin V.V., Ufimtsev K.G., Kolotyrkina N.G., Alekseyev I.N., Patov S.A. Khimiya rastitel'nogo syr'ya, 2019, no. 4, pp. 243–248. DOI: 10.14258/jcprm.2019045133 (in Russ.).
Waltho J.P., Williams D.H., Mahato S.B., Pal B.C., Barna J.C.J. J. Chem. Soc. Perkin Trans., 1986, vol. 8, no. 1, pp. 1527–1531.
Lavaud C., Massiot G., Barrera G.B., Moretti Ch., Le Men-Oliver L. Phytochemistry, 1994, vol. 37, no. 6, pp. 1671–1677.
Jansakul C., Baumann H., Kenne L., Samuelsson G. Planta Med., 1987, vol. 53, pp. 405–409.
Reznicek G., Jorenitsch J., Robien W., Kubelka W. Phytochemistry, 1989, vol. 28, no. 3, pp. 825–828.
Park J.H., Kwak J.H., Khoo J.H., Park S.-H., Kim D.U., Ha D.M., Choi S.U., Kang S.C., Zee O.P. Arch Pharm Res., 2010, vol. 33, no. 8, pp. 1175–1180.
Zhang D.M., Wang Y., Tang M.K., Chan Y.W., Lam H.M., Ye W.C., Fung K.P. Biochem. Biophys. Res. Commun., 2007, vol. 362, pp. 759–765.
Shyur L.-F., Tsung J.-H., Chen J.-H., Chiu C.-Y., Lo J.-P. Inter. J. Appl. Sci. Eng. Technol., 2005, vol. 3, pp. 195–202.
Kim H.-J., Chen F, Wang X, Chung H.Y., Jin Z. J. Agric. Food Chem., 2005, vol. 53, pp. 7691–7695.
Furtado J.C.N.A., Pirson L., Edelberg H., Miranda L.M., Loira-Pastoriza C., Preat V., Larondelle Y., André C.M. Mol-ecules, 2017, vol 22, no. 3, p. 400. DOI: 10.3390/molecules22030400.
Badole S.L., Zanwar A.A., Khopade A.N., Bodhankar S.L. Asian Pac. J. Trop. Med., 2011, vol 4, no. 11, pp. 910–916. DOI: 10.1016/S1995-7645(11)60217-4.
Santiago L.A., Dayrit K.C., Correa P.C.B., Mayor A.B.R. Journal of Natural Products, 2014, vol. 7, pp. 29–36.
El Hosry L., Di Giorgio C., Birer C., Habib J., Tueni M., Bun S.-S., Herbette G., De Meo M., Ollivier E., Elias R. Pharm. Biol., 2014, vol. 52, no. 9, pp. 1134–1140.
Apak R., Gorinstein S., Böhm V., Schaich K.M., Özyürek M., Güçlü K. Pure Appl. Chem., 2013, vol. 85, no. 5, pp. 957–998. DOI: 10.1351/PAC-REP-12-07-15.
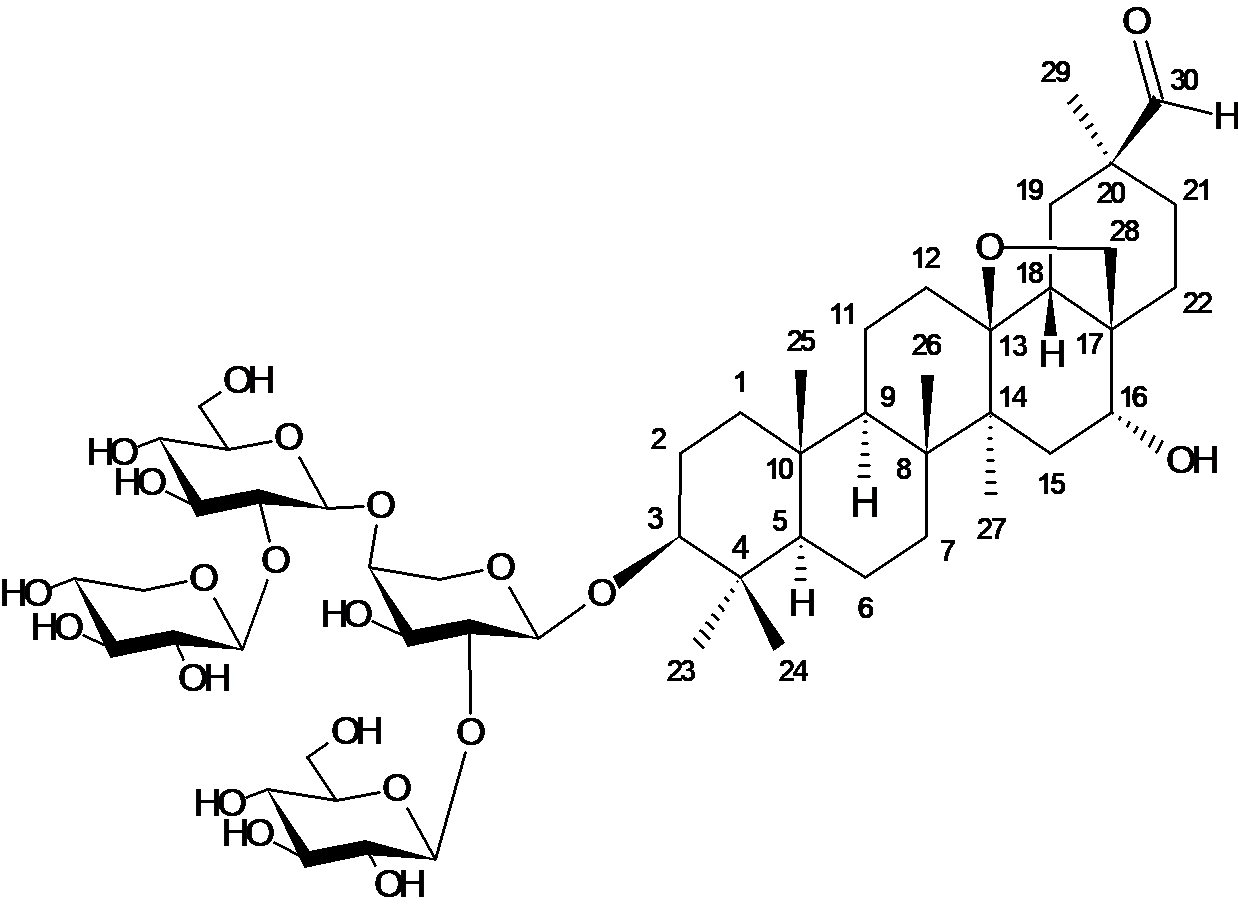
Copyright (c) 2020 chemistry of plant raw material

This work is licensed under a Creative Commons Attribution 4.0 International License.

This work is licensed under a Creative Commons Attribution 4.0 International License.
The authors, which are published in this journal, agree to the following conditions:
1. Authors retain the copyright to the work and transfer to the journal the right of the first publication along with the work, at the same time licensing it under the terms of the Creative Commons Attribution License, which allows others to distribute this work with the obligatory indication of the authorship of this work and a link to the original publication in this journal .
2. The authors retain the right to enter into separate, additional contractual agreements for the non-exclusive distribution of the version of the work published by this journal (for example, to place it in the university depository or to publish it in a book), with reference to the original publication in this journal.
3. Authors are allowed to post their work on the Internet (for example, in a university repository or on their personal website) before and during the review process of this journal, as this may lead to a productive discussion, as well as more links to this published work.











