PINUS SYLVESTRIS ETHANOL LIGNIN AND ITS AZO DERIVATIVES AS A COMPONENT OF SUNSCREEN
UDC 615.454:547.992.3:547.556.3
Abstract
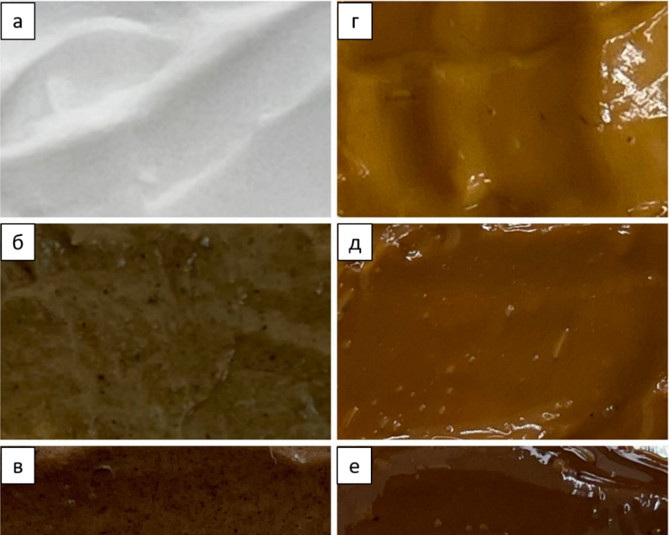
Ethanol lignin from pine wood (Pinus sylvestris) and its azo derivatives synthesized by azo coupling with diazonium salts of 4-nitroanilin and sulfanilic acid was obtained. The structure azo compounds was confirmed by IR spectroscopy, depending on the functionalization of the diazonium salt, absorption bands of nitro – or sulfo-groups appear. Due to the presence of a sulfo group, a corresponding azo derivative is soluble in water. Based on data from the elemental composition and classical ideas about lignin structure, the degree of substitution was determined: one azo group account for 3 phenylpropane units. Molecular weight of azo derivatives of lignin increased compared to initial ethanol lignin. Lignin and azo compounds were tested as photoactive components in sunscreens. The water-soluble derivative of azo is dissolved in a cream base, while insoluble modifications of ethanolignin are distributed in micron-sized particles (up to 50 micrometers). Modification by azo coupling significantly changed the color of lignin and cream that containing it. Due to the lignin modification, it was possible to achieve an increase, albeit a small one, in the absorption efficiency in the long-wavelength part of the UV-A region (320–400 nm). Developed cream samples had SPF 5–10, which corresponds to daily sunscreens.
Downloads
References
Olisova O.Yu., Vladimirova Ye.V., Babushkin A.M. Rossiyskiy zhurnal kozhnykh i veneri-cheskikh bolezney, 2012, vol. 6, no. 6, pp. 57–62. (in Russ.).
Sviridova A., Ishchenko A. Izvestiya vysshikh uchebnykh zavedeniy. Khimiya i khimicheskaya tekhnologiya, 2006, vol. 49, no. 11, pp. 3–14. (in Russ.).
Ishchenko A., Sviridova A. Izvestiya vysshikh uchebnykh zavedeniy. Khimiya i khimicheskaya tekhnologiya, 2006, vol. 49, no. 12, pp. 3–16. (in Russ.).
Sadeghifar H., Ragauskas A. Polymers, 2020, vol. 12, no. 5, 1134. https://doi.org/doi10.3390/polym12051134.
Lv S., Liang S., Zuo J., Zhang S., Wang J., Wei D. Iranian Polymer Journal, 2023, vol. 32, no. 11, pp. 1477–1497. https://doi.org/10.1007/s13726-023-01218-0.
Widsten P. Cosmetics, 2020, vol. 7, no. 4, 85. https://doi.org/10.3390/cosmetics7040085.
Barapatre A., Meena A.S., Mekala S., Das A., Jha H. International Journal of Biological Macromolecules, 2016, vol. 86, pp. 443–453. https://doi.org/10.1016/j.ijbiomac.2016.01.109.
Spiridon I., Poni P., Ghica G. Cellulose Chemistry and Technology, 2018, vol. 52, no. 7-8, pp. 543–550.
Karmanov A.P., Yermakova A.V., Raskosha O.V., Bashlykova L.A., Rachkova N.G., Kocheva L.S. Khimiya ras-titel'nogo syr'ya, 2023, no. 4, pp. 5–28. https://doi.org/10.14258/jcprm.20230412560. (in Russ.).
Antunes F., Mota I.F., Fangueiro J.F., Lopes G., Pintado M., Costa P.S. International Journal of Biological Macro-molecules, 2023, vol. 234, 123592. https://doi.org/10.1016/j.ijbiomac.2023.123592.
Qian Y., Zhong X., Li Y., Qiu X. Industrial Crops and Products, 2017, vol. 101, pp. 54–60. https://doi.org/10.1016/j.indcrop.2017.03.001.
Gordobil O., Olaizola P., Banales J.M., Labidi J. Molecules, 2020, vol. 25, no. 5, 1131. https://doi.org/10.3390/molecules25051131.
Widsten P., Tamminen T., Liitiä T. ACS Omega, 2020, vol. 5, no. 22, pp. 13438–13446. https://doi.org/10.1021/acsomega.0c01742.
Duy N.V., Tsygankov P.Y., Menshutina N.V. ChemEngineering, 2024, vol. 8, no. 4, 69. https://doi.org/10.3390/chemengineering8040069.
Lyu Y., Ji X.-X., Tian Z., Ji H., Zhang F., Dai L., Xie H., Si C. International Journal of Biological Macromolecules, 2023, vol. 230, 123122. https://doi.org/10.1016/j.ijbiomac.2022.123122.
Zhang H., Liu X., Fu S., Chen Y. Industrial & Engineering Chemistry Research, 2019, vol. 58, no. 31, pp. 13858–13867. https://doi.org/10.1021/acs.iecr.9b02086.
Zhang H., Bai Y., Yu B., Liu X., Chen F. Green Chemistry, 2017, vol. 19, no. 21, pp. 5152–5162. https://doi.org/10.1039/C7GC01974B.
Zhang J., Tian Z., Ji X.-X., Zhang F. International Journal of Biological Macromolecules, 2023, vol. 231, 123244. https://doi.org/10.1016/j.ijbiomac.2023.123244.
Wang B., Sun D., Wang H.-M., Yuan T.-Q., Sun R.-C. ACS sustainable chemistry & engineering, 2019, vol. 7, no. 2, pp. 2658–2666. https://doi.org/10.1021/acssuschemeng.8b05735.
Girard V., Fragnières L., Chapuis H., Brosse N., Marchal-Heussler L., Canilho N., Parant S., Ziegler-Devin I. Poly-mers, 2024, vol. 16, no. 13, 1901. https://doi.org/10.3390/polym16131901.
de Araújo Padilha C.E., da Costa Nogueira C., Oliveira Filho M.A., de Santana Souza D.F., de Oliveira J.A., dos San-tos E.S. Process Biochemistry, 2020, vol. 91, pp. 23–33. https://doi.org/10.1016/j.procbio.2019.11.029.
Wu Y., Qian Y., Lou H., Yang D., Qiu X. ACS Sustainable Chemistry & Engineering, 2019, vol. 7, no. 19, pp. 15966–15973. https://doi.org/10.1021/acssuschemeng.9b02317.
Zhang H., Liu X., Fu S., Chen Y. International journal of biological macromolecules, 2019, vol. 133, pp. 86–92. https://doi.org/10.1016/j.ijbiomac.2019.04.092.
Wu Y., Wu X., Zhang A., Ouyang X., Lou H., Yang D., Qian Y., Qiu X. Advanced Functional Materials, 2023, vol. 33, no. 43. 2303889. https://doi.org/10.1002/adfm.202303889.
Wu Y., Wu X., Zhang A., Ouyang X., Lou H., Yang D., Qian Y., Qiu X. Advanced Functional Materials, 2023, vol. 33, no. 43, 2303889. https://doi.org/10.1002/adfm.202303889.
Gogotov A., Luzhanskaya I. Khimiya rastitel'nogo syr'ya, 2005, no. 4, pp. 5–24. (in Russ.).
Gogotov A. Khimiya rastitel'nogo syr'ya, 1999, no. 1, pp. 39–52. (in Russ.).
Golubkov V.A., Tarabanko V.E., Kaygorodov K.L., Chelbina Y.V., Shestakov S.L., Smirnova M.A., Popov A.A., Skripnikov A.M., Vigul D.O., Borovkova V.S. Khimiya rastitel'nogo syr'ya, 2023, no. 4, pp. 137–145. https://doi.org/10.14258/jcprm.20230413782.
Kozhevnikov A.Yu., Shestakov S., Sypalova Yu.A. Khimiya rastitel'nogo syr'ya, 2023, no. 2, pp. 5–26. https://doi.org/10.14258/jcprm.20230211737. (in Russ.).
Golubkov V.A., Borovkova V.S., Lutoshkin M.A., Zos'ko N.A., Vasilieva N.Y., Malyar Y.N. Wood Science and Technology, 2024, vol. 58, no. 5-6, pp. 1861–1879. https://doi.org/10.1007/s00226-024-01590-x.
Borovkova V.S., Malyar Y.N., Vasilieva N.Y., Skripnikov A.M., Ionin V.A., Sychev V.V., Golubkov V.A., Taran O.P. Materials, 2023, vol. 16, no. 4, 1525. https://doi.org/10.3390/ma16041525.
Michelin M., Liebentritt S., Vicente A.A., Teixeira J.A. International Journal of Biological Macromolecules, 2018, vol. 120, pp. 159–169. https://doi.org/10.1016/j.ijbiomac.2018.08.046.
Sypalova Y.A., Belesov A.V., Grishanovich I.A., Repina V.I., Chukhchin D.G., Kozhevnikov A.Y. International Journal of Biological Macromolecules, 2025, vol. 290, 138952. https://doi.org/10.1016/j.ijbiomac.2024.138952.
Qian Y., Qiu X., Zhu S. Green Chemistry, 2015, vol. 17, no. 1, pp. 320–324. https://doi.org/10.1039/C4GC01333F.
Copyright (c) 2025 Chemistry of plant raw material

This work is licensed under a Creative Commons Attribution 4.0 International License.

This work is licensed under a Creative Commons Attribution 4.0 International License.
The authors, which are published in this journal, agree to the following conditions:
1. Authors retain the copyright to the work and transfer to the journal the right of the first publication along with the work, at the same time licensing it under the terms of the Creative Commons Attribution License, which allows others to distribute this work with the obligatory indication of the authorship of this work and a link to the original publication in this journal .
2. The authors retain the right to enter into separate, additional contractual agreements for the non-exclusive distribution of the version of the work published by this journal (for example, to place it in the university depository or to publish it in a book), with reference to the original publication in this journal.
3. Authors are allowed to post their work on the Internet (for example, in a university repository or on their personal website) before and during the review process of this journal, as this may lead to a productive discussion, as well as more links to this published work.















