CHEMICAL CHARACTERIZATION OF NATIVE WATER-SOLUBLE POLYSACCHARIDES OF HYPERICUM PERFORATUM L., URTICA DIOICA L., FILIPENDULA ULMARIA (L.) MAXIM., SOLIDAGO VIRGAUREA L., SYMPHYTUM OFFICINALE L., SYRINGA VULGARIS L.
UDC 615.275.4
Abstract
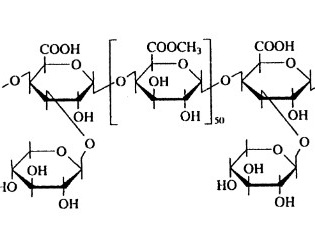
The aim was to study previously undescribed native water-soluble polysaccharides (NWSPS) of plant species used as anti-inflammatory and/or immunomodulatory agents. NWSPS are obtained with water without heating from raw materials previously purified from lipophilic and phenolic components, followed by ethanol precipitation and purification by dialysis. The protein and uronic acid content were determined by spectrophotometric method, the molecular mass characteristics by high-efficiency exclusion chromatography, the monosaccharide composition by gas chromatography, and infrared spectra were obtained. Neutral sugars are the predominant components of all the studied NWSPS. The retention of uronic acids ranged from 5–7% (Filipendula ulmaria, Solidago virgaurea, Symphytum officinale) to 14–15% (Hypericum perforatum, Urtica dioica, Syringa vulgaris); protein – about 7–10% (with the exception of S. vulgaris). All the studied species have NWSPS that are homogeneous in molecular weight and have a low coefficient of heterogeneity (with the exception of S. virgaurea); they contain 1-6 fragments with a molecular weight from 100 to 400 kDa and 24-29 fragments with a molecular weight from 10 to 100 kDa. Monomeric fragments of H. perforatum NWSPS are represented in equal parts by xylose and galacturonic acid; U. dioica – xylose>glucose>galacturonic acid; F. ulmaria – xylose>rhamnose; S. virgaurea – glucose>galactose and galacturonic acid>xylose; S. officinale – glucose>xylose and galacturonic acid; S. vulgaris – galactose >galacturonic acid>xylose and glucose. Pyranose and furanose forms of monosaccharide fragments, α- and β-type glycosidic bonds are present in all NWSPS. It seems promising to further investigate the anti-inflammatory and/or immunomodulatory activity of the described NWSPS in order to justify further in-depth investigation of the chemical composition of polysaccharide complexes with proven biological activity.
Downloads
References
Wang M., Li C., Li J., Hu W., Yu A., Tang H., Li J., Kuang H., Zhang H. Molecules, 2023, vol. 12, no. 28, 4813. https://doi.org/10.3390/molecules28124813.
Chen H., Sun J., Liu J., Gou Y., Zhang X., Wu X., Sun R., Tang S., Kan J., Qian C., Zhang N., Jin C. Int. J. Biol. Macromol., 2019, vol. 131, pp. 484–494. https://doi.org/10.1016/j.ijbiomac.2019.03.126.
Ramberg J.E., Nelson E.D., Sinnott R.A. Nutrition Journal, 2010, vol. 9, no. 1, 54. https://doi.org/10.1186/1475-2891-9-54.
Sokolov S.Ya., Zamotayev I.P. Spravochnik po lekarstvennym rasteniyam (Fitoterapiya). [Handbook of medicinal plants (Phytotherapy)]. Moscow, 1990, 428 p. (in Russ.).
Zlobin A.A., Martinson Ye.A., Ovechkina I.A., Durnev Ye.A., Ovodova R.G., Litvinets S.G. Khimiya rastitel'nogo syr'ya, 2011, no. 1, pp. 33–38. (in Russ.).
Heydarian M., Jooyandeh H., Nasehi B., Noshad M. Int. J. Biol. Macromol., 2017, vol. 104 (A), pp. 287–293. https://doi.org/10.1016/j.ijbiomac.2017.06.049.
Shang H., Zhou H., Duan M., Li R., Wu H., Lou Y. Int. J. Biol. Macromol., 2018, vol. 112, pp. 889–899. https://doi.org/10.1016/j.ijbiomac.2018.01.198.
Kalinkina O.V., Sychev I.A. Potentsial sovremennoy nauki, 2017, vol. 27, no. 1, pp. 60–63. (in Russ.).
Kalinkina O.V., Sychev I.A. Vestnik Tverskogo gosudarstvennogo universiteta. Seriya: Biologiya i ekologiya, 2017, no. 1, pp. 62–68. (in Russ.).
Kopyt'ko Ya.F., Lapinskaya Ye.S., Sokol'skaya T.A. Khimiko-farmatsevticheskiy zhurnal, 2011, vol. 45, no. 10, pp. 32–41. https://doi.org/10.30906/0023-1134-2011-45-10-32-41. (in Russ.).
Olennikov D.N., Kashchenko N.I., Chirikova N.K. Molecules, 2017, vol. 22, 16. https://doi.org/10.3390/molecules22010016.
Patent 2735080 (RU). 2020. (in Russ.).
Reshetov Ya.Ye., Ligachova A.A., Avdeyeva Ye.Yu., Danilets M.G., Golovchenko V.V., Trofimova Ye.S., Gulina Ye.I., Sherstoboyev Ye.Yu., Gur'yev A.M., Rovkina K.I., Krivoshchekov S.V., Belousov M.V. Khimiya rastitel'nogo syr'ya, 2019, no. 4, pp. 77–85. https://doi.org/10.14258/jcprm.2019045483. (in Russ.).
Gosudarstvennaya farmakopeya Rossiyskoy Federatsii. XIV izdaniye. T. 1: Vvedeniye. Obshchiye polozheniya. Me-tody analiza lekarstvennykh sredstv. Reaktivy. [State Pharmacopoeia of the Russian Federation. XIV edition. Vol. 1: In-troduction. General Provisions. Methods of Analysis of Medicines. Reagents]. Moscow, 2018, 1814 p. (in Russ.).
Dubois M., Gilles K.A., Hamilton J.K., Rebers P.A., Smith F. Anal. Chem., 1956, vol. 28, pp. 350–356. https://doi.org/10.1021/ac60111a017.
Chen H.X., Zhang M., Qu Z.S., Xie B.J. Food Chem., 2008, vol. 106, pp. 559–563. https://doi.org/10.1016/j.foodchem.2007.06.040.
Li X.Y., Wang L., Wang Y., Xiong Z.H. Effect of drying method on physicochemical properties and antioxidant activi-ties of Hohenbuehelia serotina polysaccharides // Process Biochem. 2016, vol. 51. Рp. 1100–1108. https://doi.org/10.1016/j.procbio.2016.05.006.
Kong L.S., Yu L., Feng T., Yin X.J., Liu T.J., Dong L. Carbohydr. Polym., 2015, vol. 125, pp. 1–8. https://doi.org/10.1016/j.carbpol.2015.02.042.
Gan L., Hua Z.S., Liang Y., Bi X.H. Int. Immunopharmacol., 2004, vol. 4, pp. 563–569. https://doi.org/10.1016/j.intimp.2004.01.023.
Schepetkin I.A., Quinn M.T. Int. Immunopharmacol., 2006, vol. 6, pp. 317–333. https://doi.org/10.1016/j.intimp.2005.10.005.
Song J.Y., Yang H.O., Pyo S.N., Jung I.S., Yi S.Y., Yun Y.S. Arch. Pharm. Res., 2002, vol. 25, pp. 158–164. https://doi.org/10.1007/BF02976557.
Ma G., Yang W., Mariga A.M., Fang Y., Ma N., Pei F., Hu Q. Sci. Rep., 2014, vol. 114, pp. 297–305. https://doi.org/10.1016/j.carbpol.2014.07.069.
Liu J., Wen X., Zhang X., Pu H., Kan J., Jin C. Int. J. Biol. Macromol., 2015, vol. 72, pp. 1182–1190. https://doi.org/10.1016/j.ijbiomac.2014.08.058.
Kačuráková M., Capek P., Sasinková V., Wellner N., Ebringerová A. Carbohydr. Polym., 2000, vol. 43, pp. 195–203. https://doi.org/10.1016/S0144-8617(00)00151-X.
Copyright (c) 2025 Chemistry of plant raw material

This work is licensed under a Creative Commons Attribution 4.0 International License.

This work is licensed under a Creative Commons Attribution 4.0 International License.
The authors, which are published in this journal, agree to the following conditions:
1. Authors retain the copyright to the work and transfer to the journal the right of the first publication along with the work, at the same time licensing it under the terms of the Creative Commons Attribution License, which allows others to distribute this work with the obligatory indication of the authorship of this work and a link to the original publication in this journal .
2. The authors retain the right to enter into separate, additional contractual agreements for the non-exclusive distribution of the version of the work published by this journal (for example, to place it in the university depository or to publish it in a book), with reference to the original publication in this journal.
3. Authors are allowed to post their work on the Internet (for example, in a university repository or on their personal website) before and during the review process of this journal, as this may lead to a productive discussion, as well as more links to this published work.















