СИНТЕЗ И АНТИРАДИКАЛЬНАЯ АКТИВНОСТЬ ПРОСТРАНСТВЕННО ЗАТРУДНЕННЫХ ФЕНОЛЬНЫХ ПРОИЗВОДНЫХ ЛЬНЯНОЙ ЦЕЛЛЮЛОЗЫ
УДК 661.728.89
Аннотация
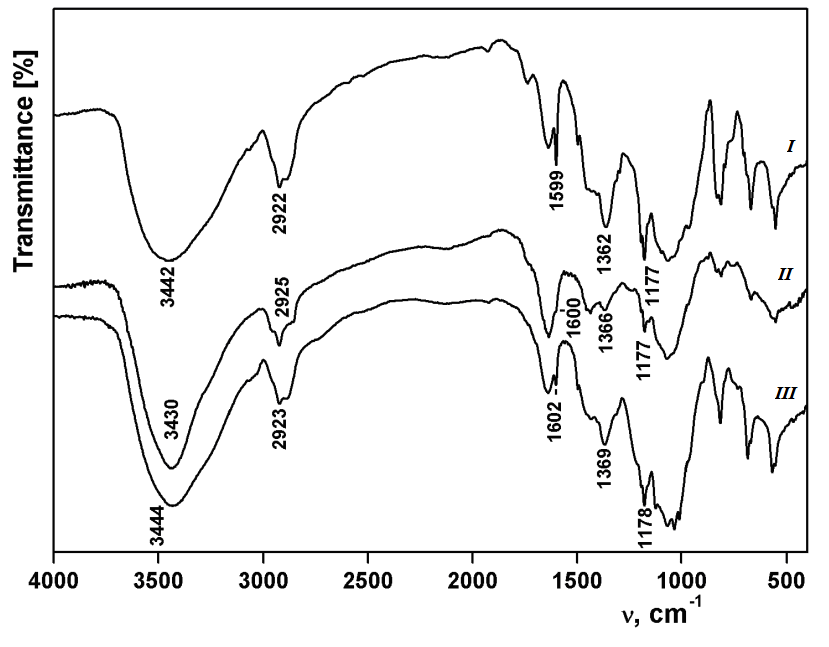
Осуществлена модификация порошковой ультрадисперсной льняной целлюлозы пространственно затрудненными фенольными фрагментами. Модификация проведена путем взаимодействия тозилата целлюлозы с гидразидом 3(3',5'-ди-трет-бутил-4'-гидроксифенил)пропионовой кислоты и с 3,5-ди-трет-бутил-4-гидроксибензилдиметиламином в среде ДМФА при 100 ○С в течение 16–30 ч. Образцы модифицированной целлюлозы охарактеризованы методами ИК- и ЯМР1 Н спектроскопии. Из данных элементного анализа рассчитана степень замещения производных целлюлозы пространственно затрудненными фенольными фрагментами. Определена антирадикальная активность полученных образцов в их реакциях со стабильным радикалом – 2,2-дифенил-1-пикрилгидразилом (ДФПГ). Реакции модифицированной целлюлозы с ДФПГ проводили в ДМСО в условиях псевдопервого порядка по радикалу. Из значений эффективных констант были рассчитаны константы скорости второго порядка. Установлено, что модификация целлюлозы пространственно затрудненными фенольными фрагментами приводит к резкому увеличению ее антирадикальной активности, которая зависит от степени замещения образца и способа замещения. Различие в активности гидразидного и бензильного производных целлюлозы может свидетельствовать об их различной пространственной структуре, обусловливающей разную доступность фенольных фрагментов. Антирадикальная активность гидразидного производного целлюлозы превышает аналогичный показатель антиоксиданта ионола (2,6-ди-трет-бутил-4-метилфенола).
Скачивания
Литература
Men'shchikova Ye.B., Lankin V.Z., Zenkov N.K., Bondar' I.A., Krugovykh N.F., Trufakin V.A.. Okislitel'nyy stress. Prooksidanty i antioksidanty. [Oxidative stress. Prooxidants and antioxidants]. Moscow, 2006, 554 p. (in Russ.).
Aref'yev D.V., Belostotskaya I.S., Vol'yeva V.B., Domnina N.S., Komissarova N.L., Sergeyeva O.Yu., Khrustaleva R.S. Izvestiya Akademii Nauk, seriya khimiya, 2007, no. 4, pp. 751–761. DOI: 10.1007/s11172-007-0117-x. (in Russ.).
Torlopov M.A., Chukicheva I.Yu., Kuchin A.V. Khimiya prirodnykh soyedineniy, 2011, vol. 47, no. 6, pp. 761–763. (in Russ.).
Patent 2497828 (RU). 2013. (in Russ.).
Patent 2619934 (RU). 2017. (in Russ.).
Autlov S.A., Bazarnova N.G., Kushnir Ye.Yu. Khimiya Rastitel'nogo Syr'ya, 2013, no. 3, pp. 33–41. DOI: 10.14258/jcprm.1303033. (in Russ.).
Savel'yeva Ye.Ye., Dosadina E.E., Belov A.A. Uspekhi v khimii i khimicheskoy tekhnologii, 2016, vol. 30, no. 9, pp. 16–18. (in Russ.).
Savel'yeva Ye.Ye., Dosadina E.E., Belov A.A. Uspekhi v khimii i khimicheskoy tekhnologii, 2017, vol. 31, no. 9, pp. 14–16. (in Russ.).
Patent 2554629 (RU). 2015. (in Russ.).
Qu J., Khan F. Z., Satoh M., Wada J., Hayashi H., Mizoguchi K., Masuda T. Polymer, 2008, vol. 49, pp. 1490–1496. DOI: 10.1016/j.polymer.2008.01.065.
Yang T., Xiao P., Zhang J.M, Jia R., Nawaz H., Chen Zh., Zhang J. ACS Appl. Mater. Interfaces, 2019, vol. 11, no. 4, pp. 4302–4310. DOI: 10.1021/acsami.8b15642.
Burlakova Ye.B. Rossiyskiy khimicheskiy zhurnal, 2007, vol. LI, no. 1, pp. 3–12. (in Russ.).
Patent 2343240 (RU). 2009. (in Russ.).
Kramer J.B., Boschelli D.H., Connor D.T. J. Heterocyclic. Chem., 1994, vol. 31, pp. 1439–1443. DOI: 10.1002/jhet.5570310625.
Rahn K., Diamantoglou M., Klemm D., Berghmans H., Heinze T. Angewandte Makromolekulare Chemie, 1996, vol. 238, no. 1, pp. 143–163. DOI: 10.1002/apmc.1996.052380113.
Tkacheva N.I., Morozov S.V., Grigor'yev I.A., Mognonov D.M., Kolchanov N.A. Vysokomolekulyarnyye soyedineni-ya, seriya B, 2013, vol. 55, no. 8, pp. 1086–1107. DOI: 10.7868/S0507547513070179. (in Russ.).
Shipina O.T., Garayeva M.R., Aleksandrov A.A. Vestnik KTU, 2009, no. 6, pp. 148–152. (in Russ.).
Arzamanova I.G., Logvinenko P.M., Gurevich Ya.A. Zhurnal fizicheskoy khimii, 1973, vol. 47, no. 3, pp. 707–708. (in Russ.).
Arzamanova I.G., Nayman M.I., Gurevich Ya.A. Zhurnal fizicheskoy khimii, 1979, vol. 53, no. 4, pp. 1007–1009. (in Russ.).
Copyright (c) 2020 Химия растительного сырья

Это произведение доступно по лицензии Creative Commons «Attribution» («Атрибуция») 4.0 Всемирная.

This work is licensed under a Creative Commons Attribution 4.0 International License.
Авторы, которые публикуются в данном журнале, соглашаются со следующими условиями:
1. Авторы сохраняют за собой авторские права на работу и передают журналу право первой публикации вместе с работой, одновременно лицензируя ее на условиях Creative Commons Attribution License, которая позволяет другим распространять данную работу с обязательным указанием авторства данной работы и ссылкой на оригинальную публикацию в этом журнале.
2. Авторы сохраняют право заключать отдельные, дополнительные контрактные соглашения на неэксклюзивное распространение версии работы, опубликованной этим журналом (например, разместить ее в университетском хранилище или опубликовать ее в книге), со ссылкой на оригинальную публикацию в этом журнале.
3. Авторам разрешается размещать их работу в сети Интернет (например, в университетском хранилище или на их персональном веб-сайте) до и во время процесса рассмотрения ее данным журналом, так как это может привести к продуктивному обсуждению, а также к большему количеству ссылок на данную опубликованную работу.















